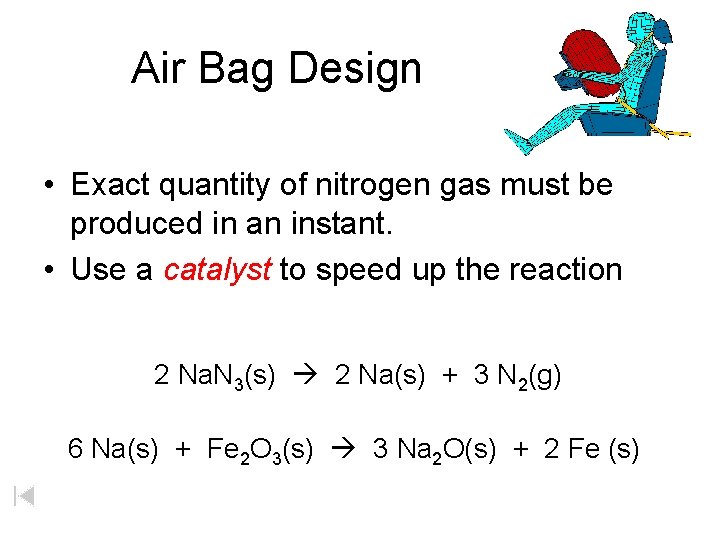Glory Air Bag Chemical Reaction

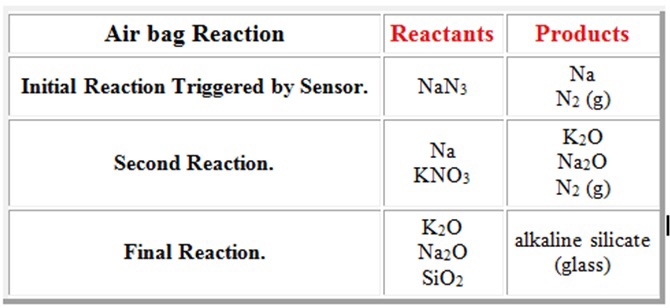
Sodium Azide Potassium Nitrate Silicon Dioxide are the initial reactants packed into the air bag module.
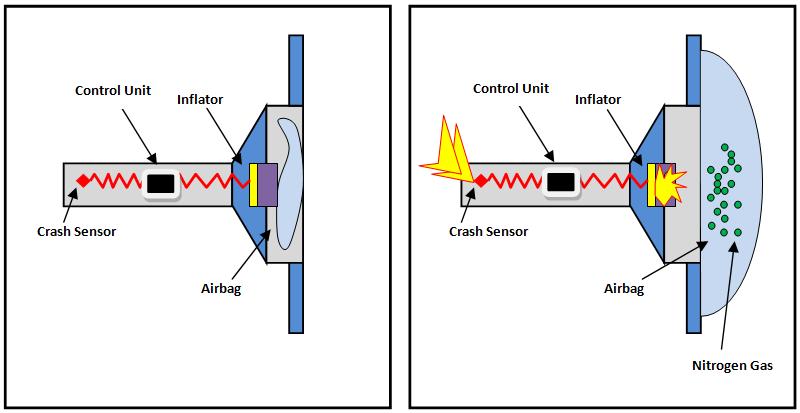
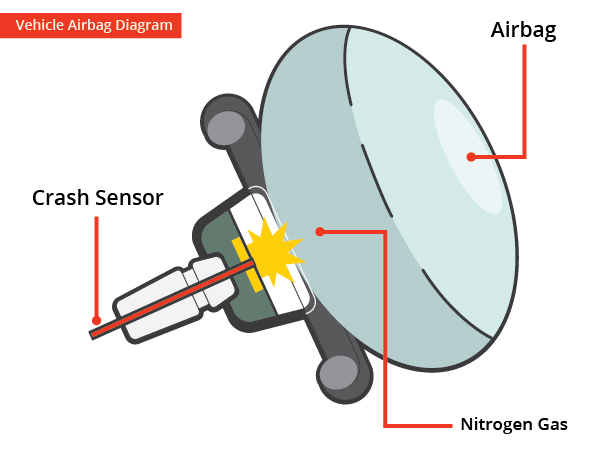
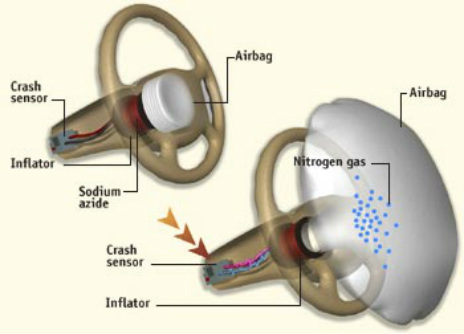
Air bag chemical reaction. A by the reaction in which they are involved reaction 1 2 or 3 and b by which side of the equation theyre on reactant or product. Air bags fitted in the majority of new automobiles are safety devices activated when a sudden deceleration causes the ignition of a propellant cartridge containing sodium azide. Airbags are meant to work in conjunction with seatbelts so buckle up.
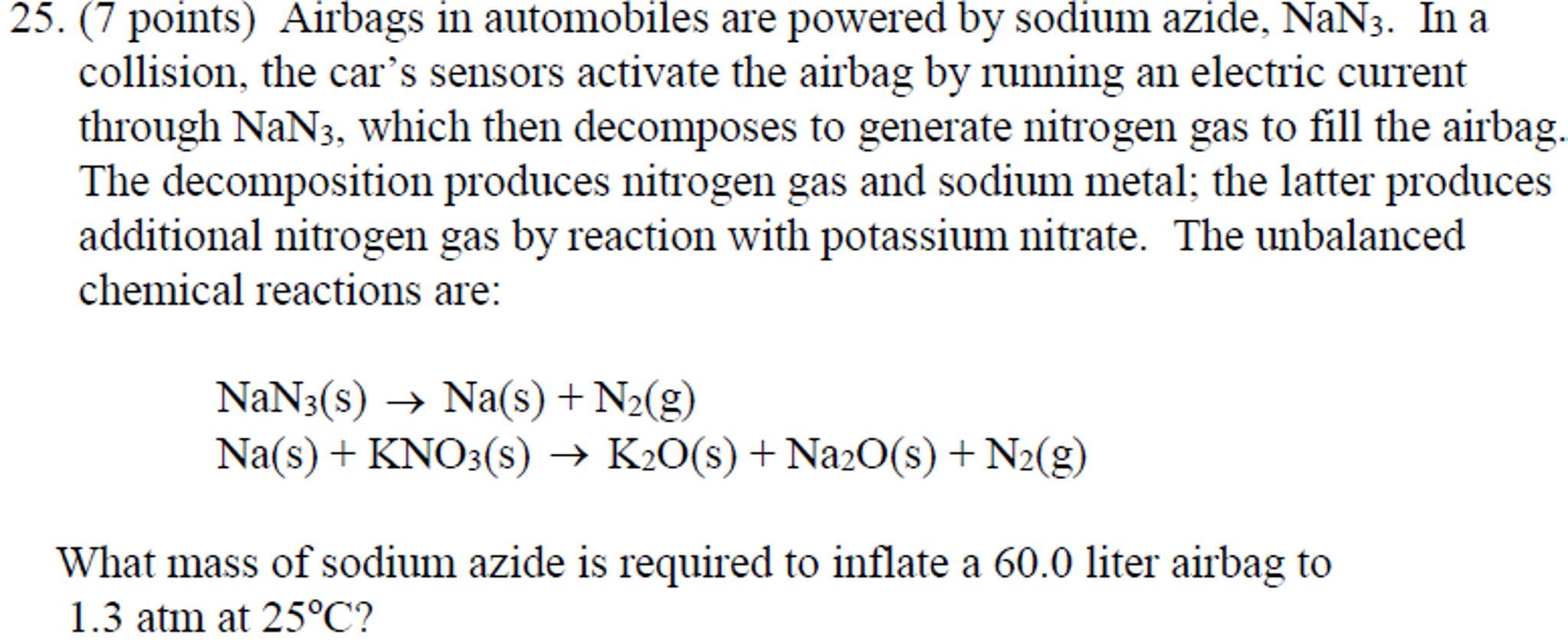
Those reactions are listed above. Sodium azide is a stable salt at ambient temperature. Reactions Science Videos May 01 2018.
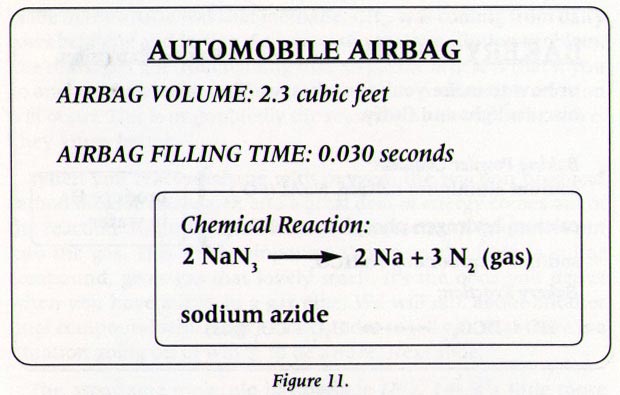
There are three reactions involved in the deployment of an air bag. The chemical reaction produces a gas that inflates the airbag the gas that the chemical reaction produces is nitrogen gas. The chemical reaction that occurs in most air bag systems is the decomposition of sodium azide NaN3 producing nitrogen an extremely unreactive gas.
The science behind the inflation of an airbag is that the airbag is inflated when it successfully goes through a chemical reaction. Chemical Reactions Used to Generate the Gas Inside the airbag is a gas generator containing a mixture of NaN3 KNO3 and SiO2. Really its the noise of an explosion.
And their chemical formulas involved in airbag deployment based on the. When sensors in the vehicle detect a collision an electrical current is passed through a carefully measured amount of NaN 3 to initiate its decomposition. Write the reaction for the decomposition of Sodium Azide.
NEW CAR ASSESSMENT PROGRAM CRASH TEST AREA CRASHES trip sensors in cars that send an electric signal to. Hot blasts of the nitrogen inflate the airbag. When the car undergoes a head-on collision a series of three chemical.