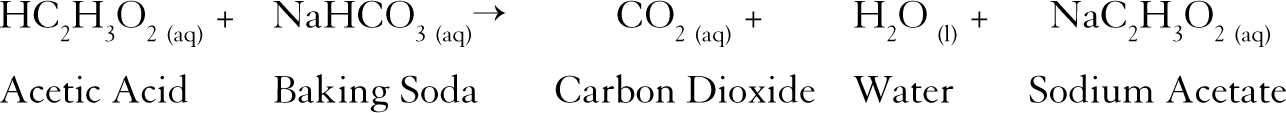Recommendation Balanced Baking Soda And Vinegar Equation

Is it OK to mix vinegar and baking soda.
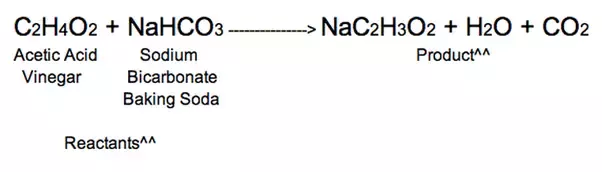
Balanced baking soda and vinegar equation. Mixing baking soda and vinegar will create a chemical reaction because one is an acid and the other a base. The equation for the reaction is. Baking soda - Chemical name sodium bicarbonate with formula NaHCO 3.
Baking soda is a bicarbonate NaHCO 3 and vinegar is an acetic acid HCH 3 COO. ZS Answer units. The overall chemical reaction between baking soda sodium bicarbonate and vinegar weak acetic acid is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas liquid water sodium ions and acetate ions.
Baking soda is a powdered chemical compound called sodium bicarbonate and vinegar includes acetic acid. Vinegar - A dilute solution of acetic acid in water. In the Answer box express your numerical answer to 2 significant figures.
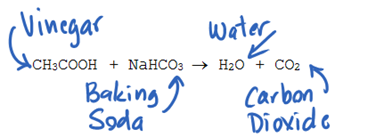
The reaction occurs once the vinegar is added to the baking soda. CH3COOH NaHCO3 CH3COONa CO2 H2O. The reaction proceeds in two steps.
Complete the table below. Baking soda sodium bicarbonate NaHCO3 and vinegar dilute acetic acid CH3COOH react to form three compounds. Balancing the equation balancing the reaction conservation of charge and mass.
Baking soda vinegar NaHCO3 aq CH3COOH aq carbon dioxide water sodium acetate CO2 g H2O l CH3COONa aq Looking closely at this equation examine whether it is balanced or not. The materials from this experiment can be disposed down the sink andor in the regular trash. In order to do this I really needed to think about using the ideal gas law to find the right amount of CO2 that would be needed and using that information and the balanced chemical equation to estimate the amounts of vinegar and baking soda needed to produce that.