Out Of This World Describe The Changes That Happen In Iron Nails When They Are Exposed In Oxygen

1 on a question.
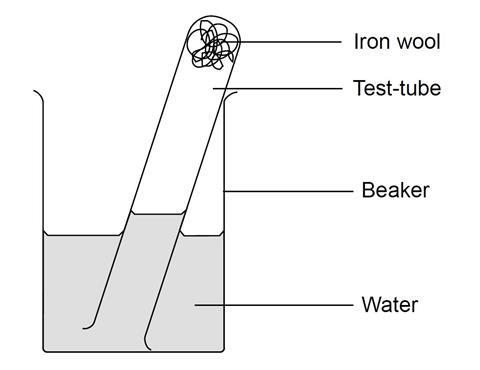
Describe the changes that happen in iron nails when they are exposed in oxygen. The oxygen and water in air react with the metal to form the hydrated oxide. 4 Fe 3 O 2 2 Fe 2 O 3. What do you call the process of exposing the iron nails placed in wet cloth after 48 hours that resulted.
In the case of iron it loses its electrons to oxygen. The iron used in this old car has the chemi cal property ofreactivity with oxygen. Iron s oxygen g iron III oxide s What is the FORMULA for the limiting reagent.
When iron is burned in oxygen or in air iron cinder forms the equation reaction is. Iron cinder is a compound in which iron has different valencies. This oxidationreduction reaction takes place via two separate half-reactions Equations.
For the following reaction 571 grams of iron are mixed with excess oxygen gas. The electrons of iron are transferred to oxygen when iron is found in the presence of oxygen. Water is essential for oxidation to.
In an oxidation reaction electrons are transferred from one substance to another. For the following reaction 650 grams of iron are mixed with excess oxygen gas. 3Fe 2O₂ Fe₃O₄ or 3Fe 2O₂ FeO Fe₂O₃.
The Chemical Reaction That Forms Rust. The familiar red form of rust is Fe 2 O 3 but iron has other oxidation states so it can form other colors of rust. When an iron nail is dipped in copper sulphate solution a brown coating of copper is formed on the surface of iron and the colour of copper sulphate solution changes.