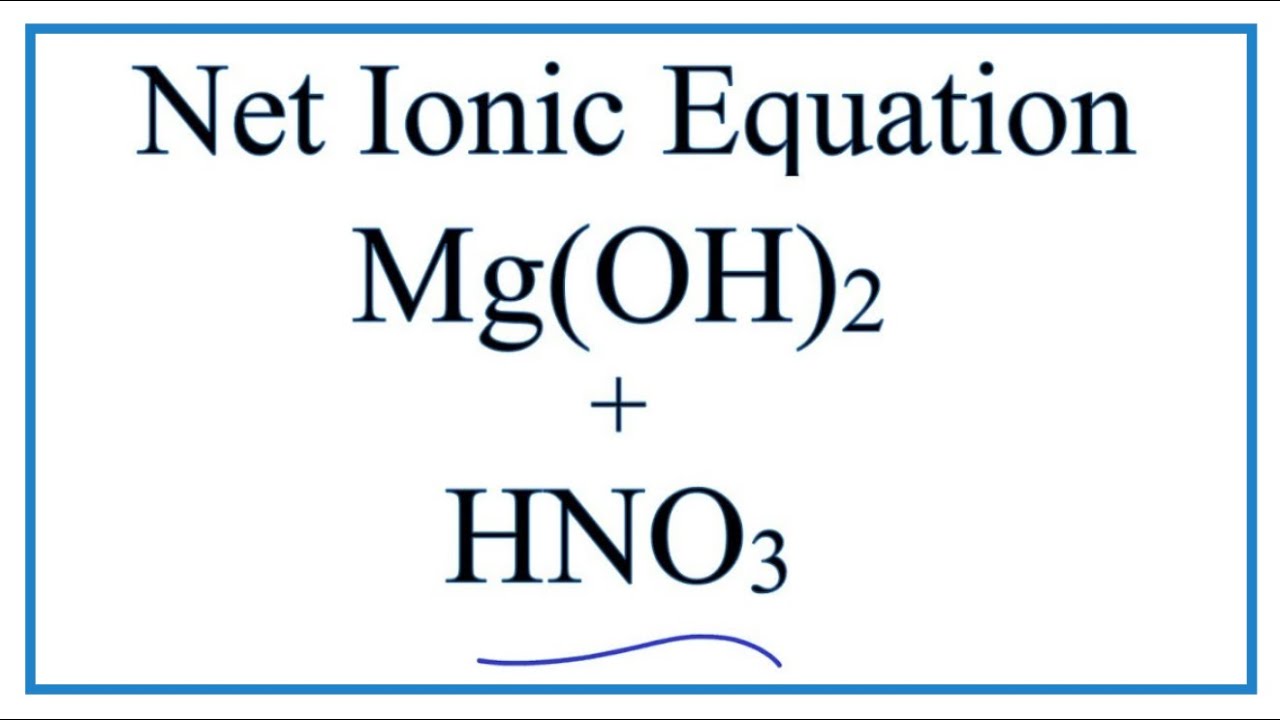Divine Nitric Acid Plus Magnesium Hydroxide

Well magnesium hydroxide is dibasic and requires 2 equiv nitric acid for equivalence.
Nitric acid plus magnesium hydroxide. H2 MgC6H6O7 As a note metal acid. Magnesium carbonate is a base and nitric acid is obviously an acid so the reaction will be an acid-base reaction and such always results in the formation of a salt and possibly other products. The most commonly used chemicals are discussed in an article available here.
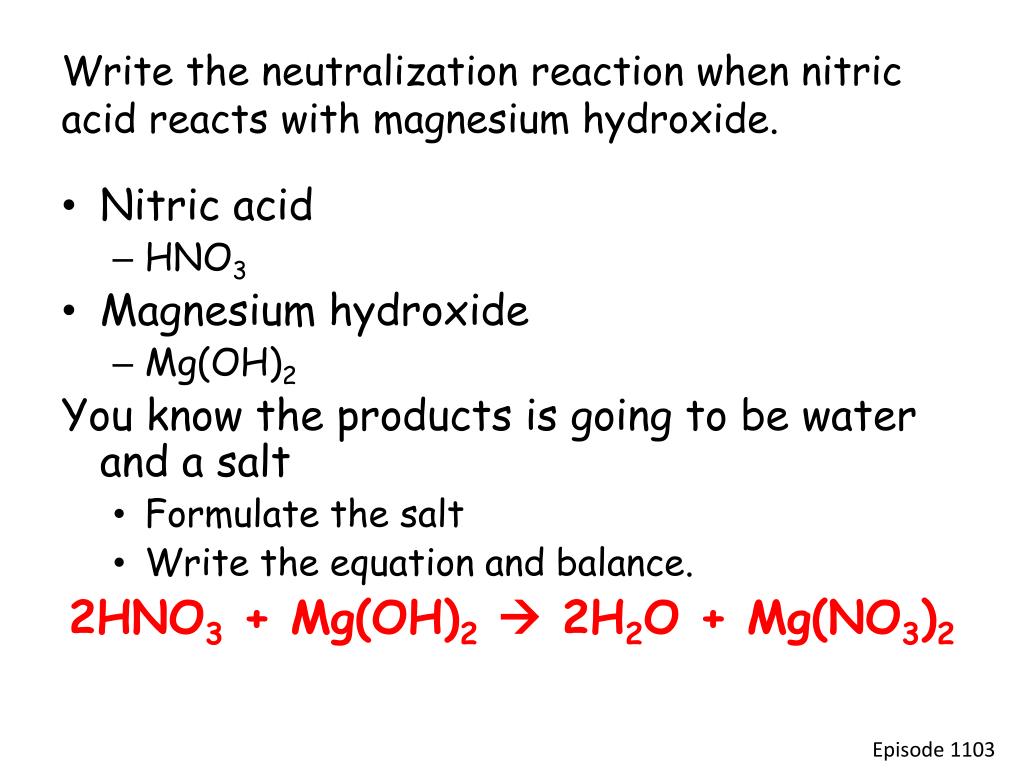
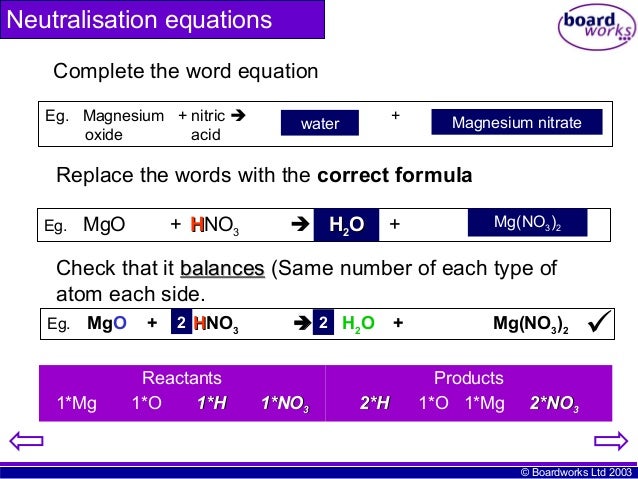
Nitric acid magnesium oxide magnesium nitrate water 2HNO3 MgO Mg NO32 H2O Also note that the reaction of metal oxides with acids is exothermic ie heat energy is given out. In this single displacement reaction the Mg replaces the H in the HNO3This is a. Be Sure To Choose The Correct Balancing Coefficients.
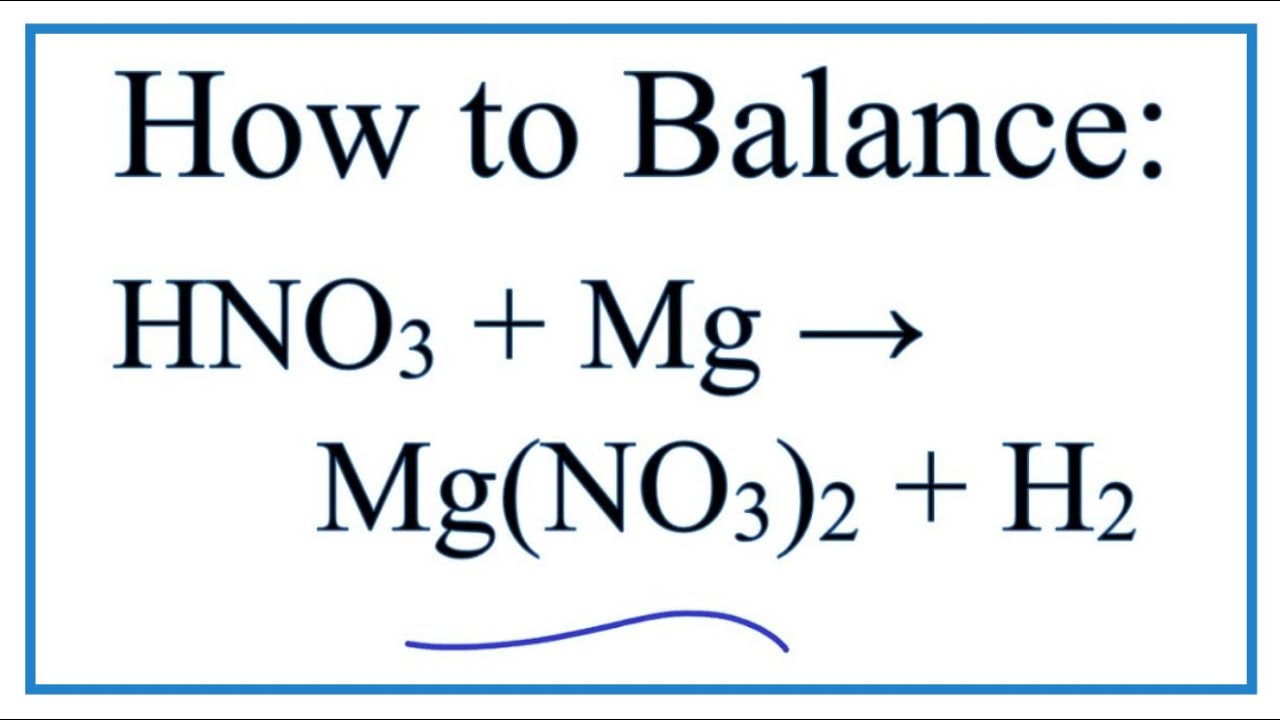
The active ingredients of an antacid tablet contained only magnesium hydroxide and aluminium hydroxide. Nitric acid Magnesium Magnesium nitrate Hydrogen gas. Sodium carbonate hydrochloric acid sodium chloride water carbon dioxide Na 2CO 3 2HCl 2NaCl H 2O CO 2 24.
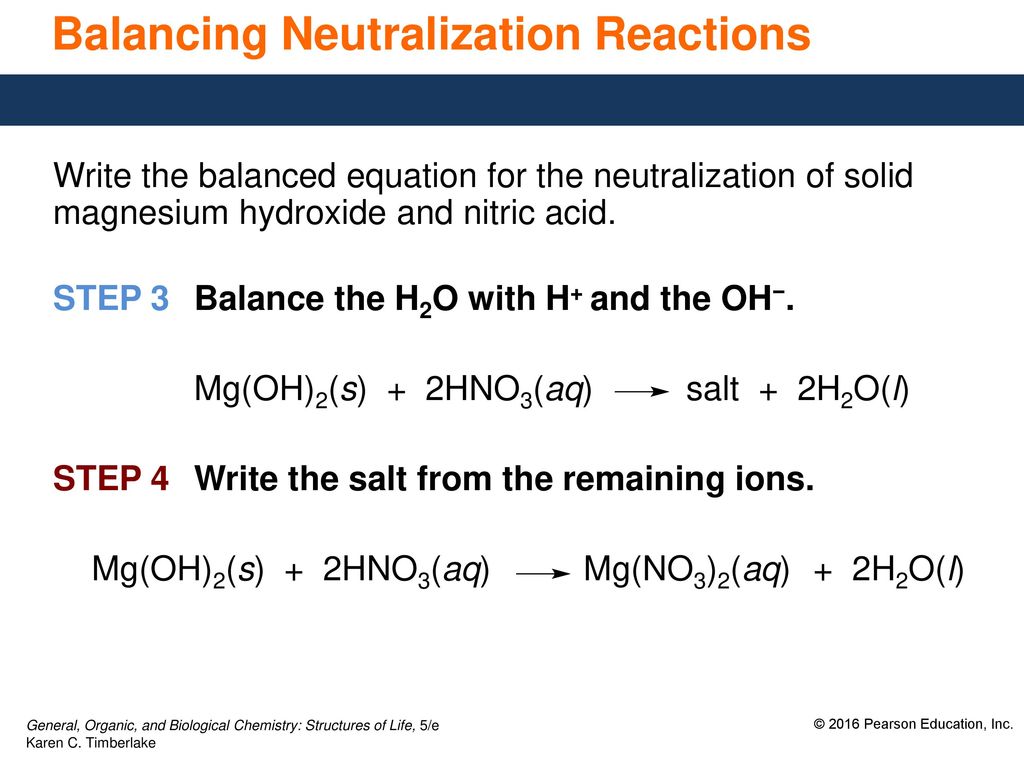
MgCO3 HNO3 Mg NO32 CO2 H2O The resulting salt is magnesium nitrate Mg NO32. Most commercially available nitric acid has a concentration of 68 in water. There are many chemicals available on the market today that are suitable for use as neutralization chemicals.
2 HNO3 MgC2H3O22 2 HC2H3O2 MgNO32 HCH2H3O22 MgNO3 2 H1 2 NO3-1 Mg2 2 C2H3O2-1 A. The magnesium hydroxide and the nitric acid combined and form a salt and water. Sulfur dioxide water sulfurous acid SO 2 H 2O H 2SO 3 23.
Learn how a hydroxide ion from a base reacts with a hydronium ion from an acid to neutralize each other and form water. Identify Any Spectator Ions. The pure compound is colorless but older samples tend to acquire a yellow cast due to decomposition into oxides of nitrogen and water.