Spectacular What Is The Balanced Chemical Equation For Vinegar And Baking Soda

Once the vinegar is added to the baking soda carbon dioxide is.
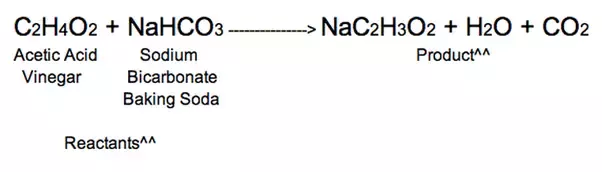
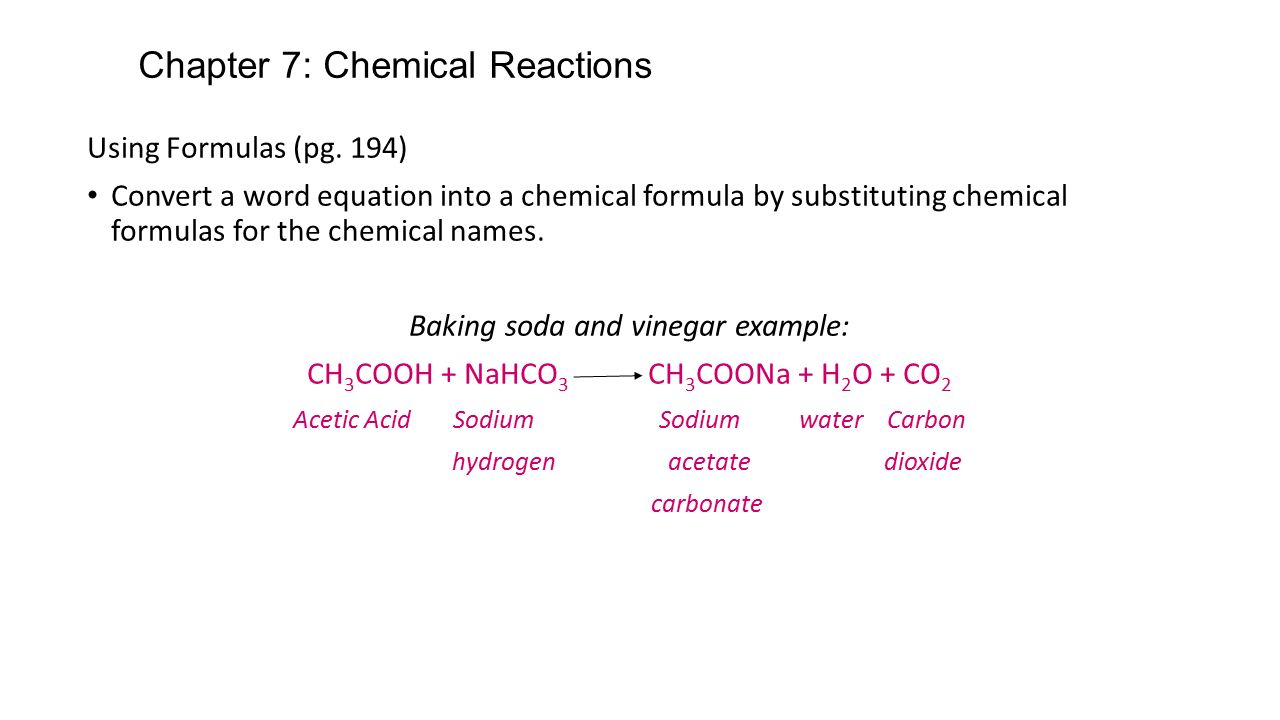
What is the balanced chemical equation for vinegar and baking soda. The first reaction is the acid -base reaction. CH3COOH NaHCO3 CH3COONa CO2 H2O. The overall chemical reaction between baking soda sodium bicarbonate and vinegar weak acetic acid is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas liquid water sodium ions and acetate ions.
What happens when baking soda is mixed with vinegar explain with the help of chemical equation. NaHCO3 aq CH3COOH aq ---- CO2 g H2O l CH3COONa aq. What is the reason why bubbling occurs when vinegar is mixed with baking soda.
When baking soda is added to vinegar the resulting reaction produces a tremendous amount of gas as shown in this video. Mgs 2 HClaq MgCl 2aq H 2g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single replacement reaction or to demonstrate the generation of hydrogen gas. Baking soda - Chemical name sodium bicarbonate with formula NaHCO 3.
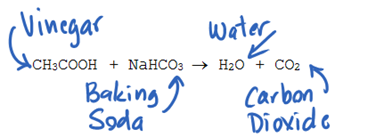
When vinegar and baking soda are first mixed together hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda. These 2 components react in solution to form carbon dioxide water and sodium acetate as shown in the chemical reaction below. Baking soda is a bicarbonate NaHCO 3 and vinegar is an acetic acid HCH 3 COO.
The Vinegar reacts with baking soda to give sodium ethanoate water along with the liberation of carbon dioxide gas. Vinegar - A dilute solution of acetic acid in water. One of the products this reaction creates is carbon dioxide.
Reaction of Vinegar with Baking Soda. Baking soda is a bicarbonate NaHCO3 and vinegar is an acetic acid HCH3COO. Baking soda is a powdered chemical compound called sodium bicarbonate and vinegar includes acetic acid.