Heartwarming Ammonia And Hydrogen Chloride Gases Are Mixed Chemical Equation

I Magnesium wire is burnt in air.
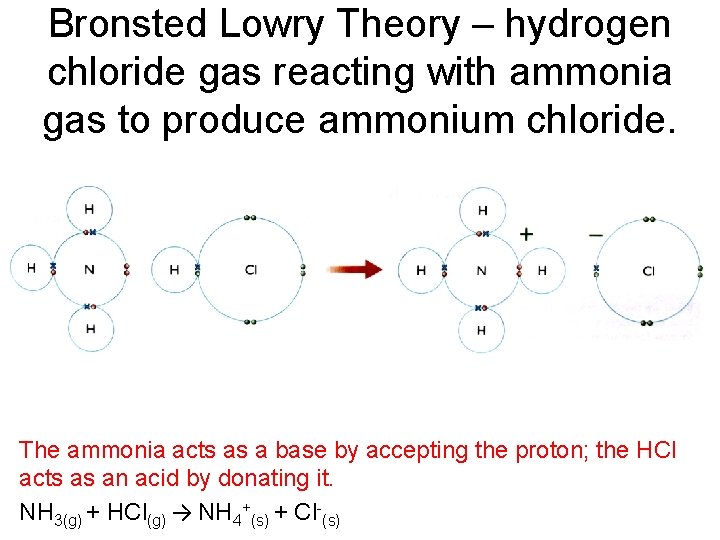
Ammonia and hydrogen chloride gases are mixed chemical equation. Ammonia is a weak base that reacts with hydrochloric acid forming a compound called ammonium chloride. Ii Electric current is passed through water. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows.
A Hydrogen gas combines with nitrogen to form ammonia. B lime-stone is heated. It also reacts with some metals to produce hydrogen gas.
Translate the following statements into chemical equations and then balance them. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows. Iii Ammonia and hydrogen chloride gasesare mixed.
Produced hydrogen chloride vapor can behave as an acidic compound can release H ions in the water. First ammonia reacts with chlorine and produce nitrogen gas and hydrogen chloride vapor. Starting early can help you score better.
HCl and NH 3 molecules diffuse through the air towards each other. Then hydrogen chloride reacts with basic ammonia gas to produce ammonium chloride which is. It is a displacement reaction.
OK when ammonia NH3 is made from hydrogen H2 and nitrogen N2 the balanced equation of the reaction is. Ammonia Gas Reacts With Chlorine Gas To Form Ammonium Chloride Solid. Avail 25 off on study pack.







.png)


