Recommendation Combustion Word Equation

S O 2 SO 2 ΔH 297 kJmol.
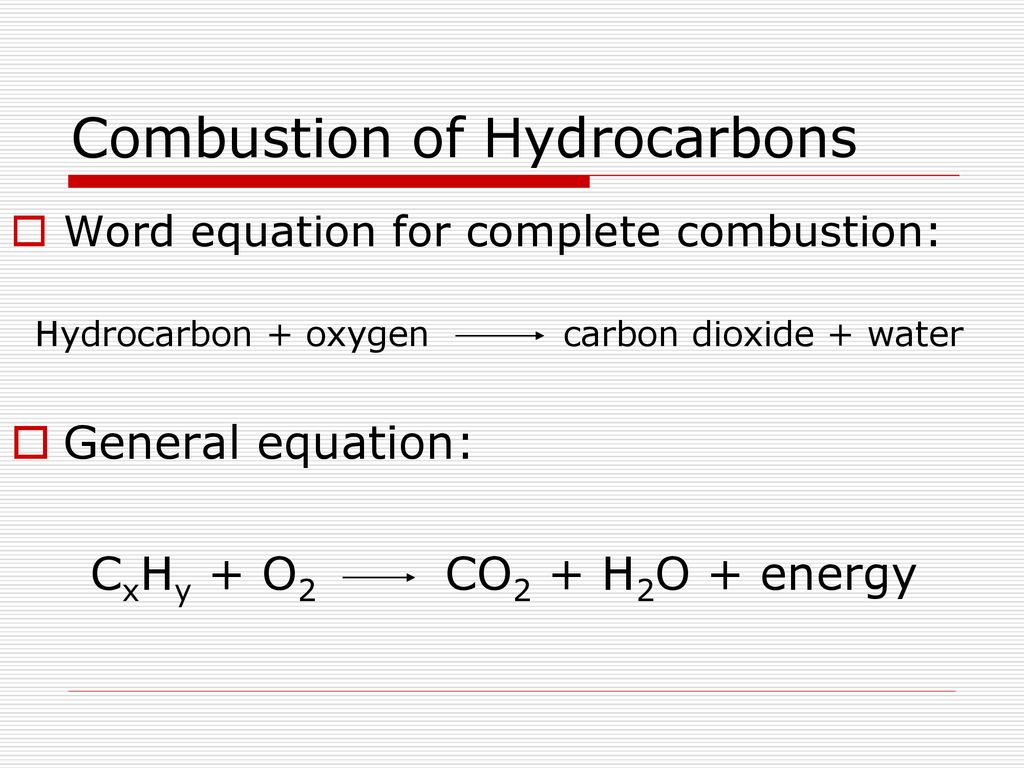
Combustion word equation. The general form of a combustion reaction can be represented by the reaction between a hydrocarbon and oxygen which yields carbon dioxide and water. General Form of a Combustion Reaction hydrocarbon oxygen carbon dioxide water Examples of Combustion Reactions Its important to remember that combustion reactions are easy to recognize because the products always contain carbon dioxide and water. C 3 H 8 5O 2 3CO 2 4H 2 O What is incomplete combustion.
The generic equation for a combustion reaction is AB O2 à AO BO where AB is a compound or molecule that is mixed with the oxygen and AO and BO are chemical compounds or molecules known as oxides since they are elements that are bonded to oxygen. Glucose is a sugar and cellulose formula is C 6 H 10 O 5 When burning wood reacts with Oxygen which is contained in air. 1 mole of octane gas 85 moles of oxygen gas 8 moles of carbon monoxide gas 9 moles of water vapour heat 21K views.
Hydrogen burns in oxygen to form water. Other cases involve burning hydrogen and oxygen without carbon and reactions that create carbon monoxide. Magnesium carbonate sulfuric acid magnesium sulfite water carbon dioxide magnesium carbonate nitric acid magnesium nitrate water carbon dioxide magnesium carbonate hydrochloric acid magnesium chloride water.
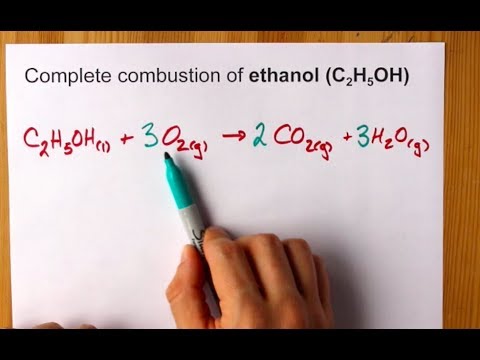
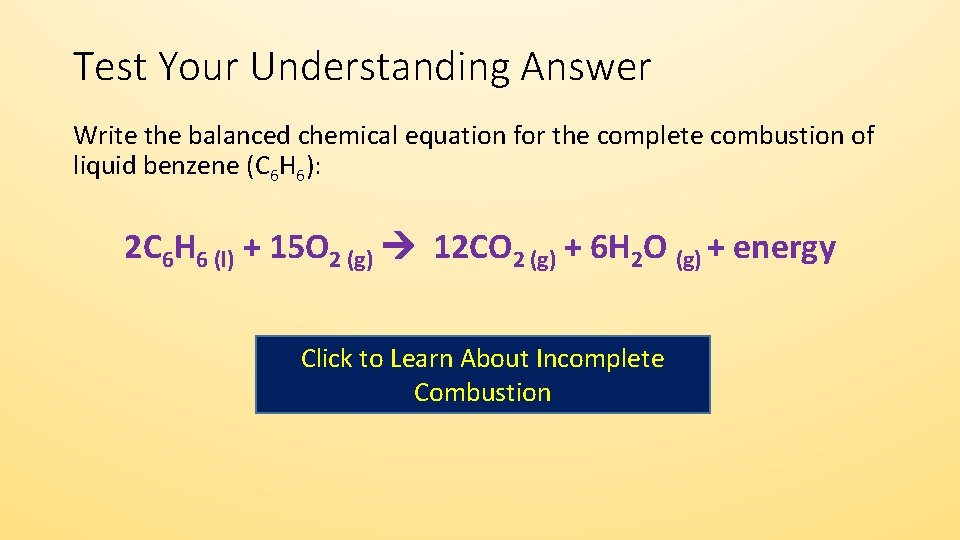
Propane oxygen carbon dioxide water. Fuel oxygen -- water _____ _____ answer choicesThe products of the complete combustion of a hydrocarbon are carbon dioxide and waterThe equation will look like this. The complete combustion of any hydrocarbon gives carbon dioxide and water.
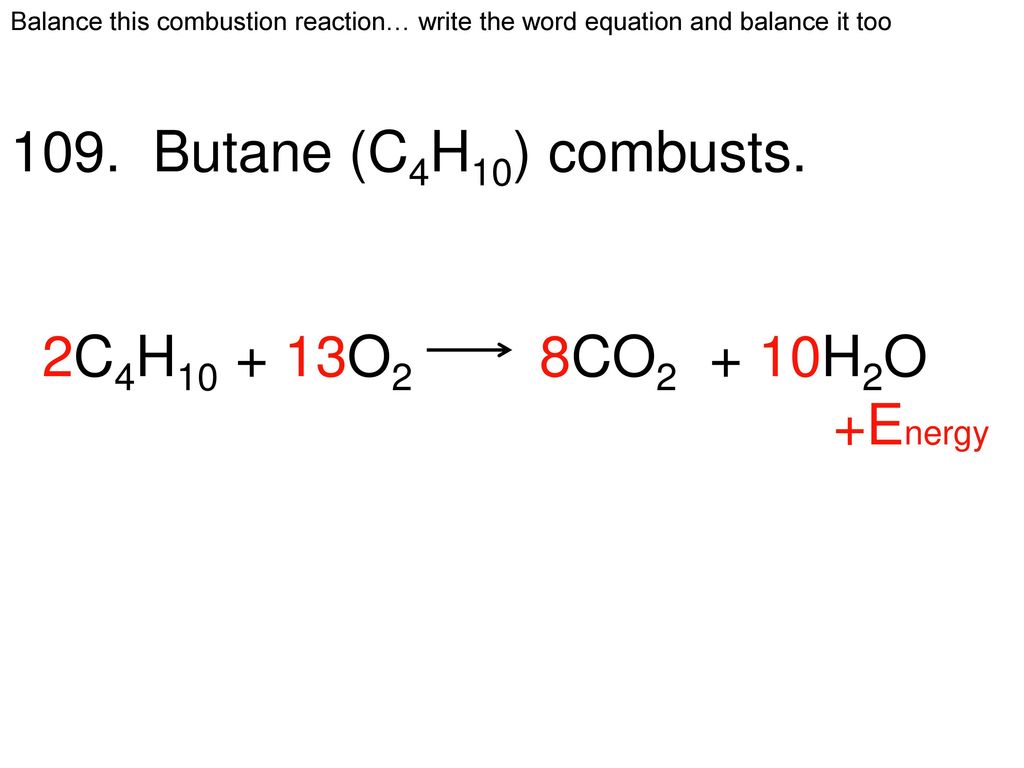
The word equation for wood combustion is that wood in the presence of oxygen and high heat combusts to produce carbon dioxide water vapor heat and ash residue. Candles are made from hydrocarbons. You just have to check the number of atoms of each element is the same in both sides.
Incomplete combustion is the burning of a hydrocarbon in insufficient oxygen producing carbon soot carbon monoxide and water as products. Hydrocarbon Oxygen Carbon dioxide water. In most cases of combustion the source of.