Supreme Skeleton Equation And Balanced Equation Difference

A skeleton equation doesnt show the relative and balanced amounts of reactants and products.
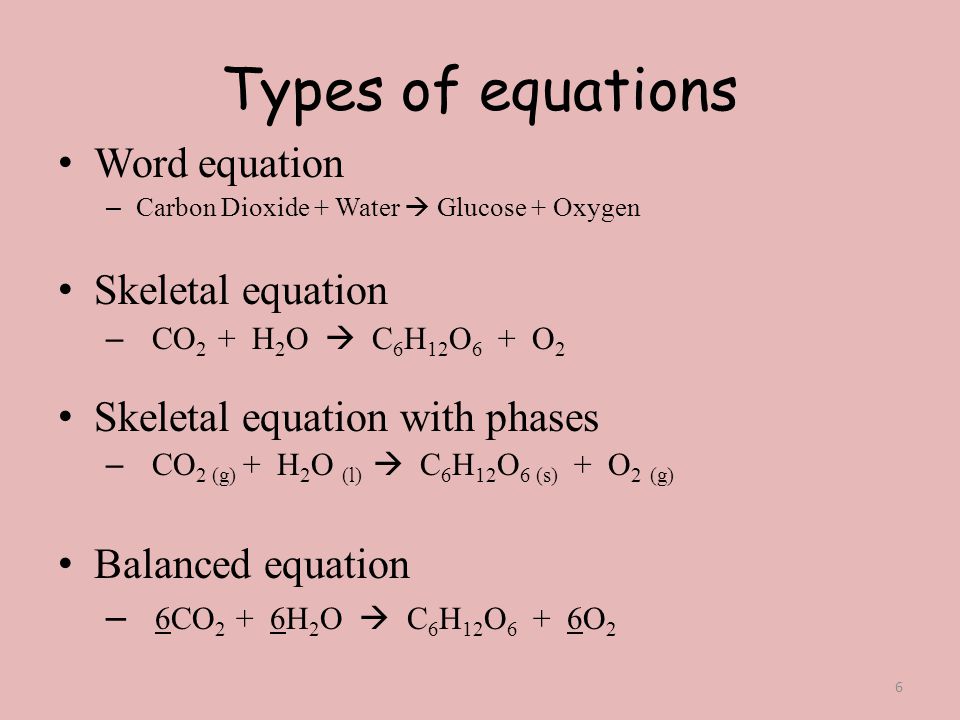
Skeleton equation and balanced equation difference. The skeleton equation is the intermediate step which enables the conversion of an equation expressed. A balanced chemical equation is that in which the total number of atoms of each element are equal on both sides of the equation is called balanced chemical equation. In skeletal chemical equation the mass no of elements in reactant side is not equal to the mass no of elements in product side.
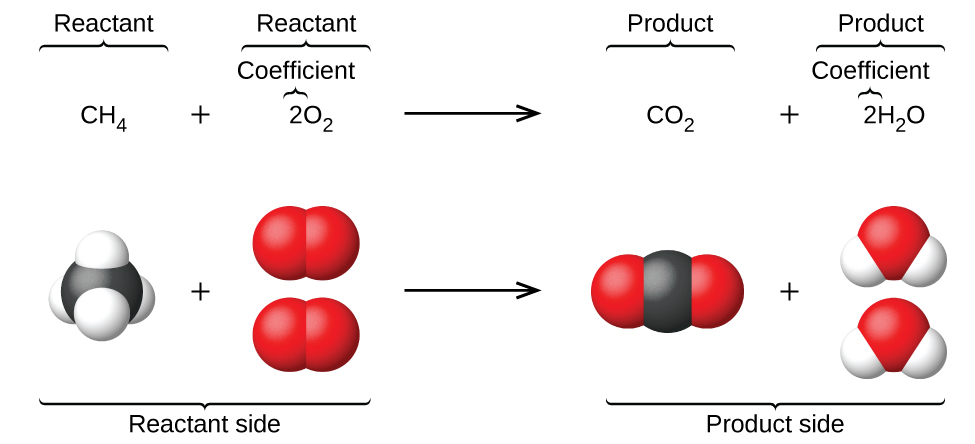
A skeleton equation is just a way of using the formulas to indicate the chemicals that were involved in the chemical reaction. In a skeleton equation you put chemical formulas in place of chemical names. A skeleton equation shows the chemical formulas and physical states of the reactants and products but it does not account for their relative amounts.
What is skeleton reaction. Skeletal chemical equation is a representation. Later it has to be balanced by appropriate number of molecules.
What is the difference between a skeleton equation and a chemical equation. For example we say hydrogen and oxygen react to produce water and we write H2 g O2 g H2O l This is a skeletal equation because it states correctly the molecules H2 and O2 and the compound H2O involved in the equation but it is not balanced. It describes a chemical reaction using the chemical formulas of the reactants and products.
A skeleton equation is just a way of using the formulas to indicate the chemicals that were involved in the chemical reaction. It shows the number of molecules and atoms of both reactants and products. This skeleton equation shows that magnesium reacts with oxygen to form magnesium oxide.
The key difference between balanced equation and skeleton equation is that balanced equation gives the actual number of molecules of each reactant and product involved in the chemical reaction whereas skeleton equation gives only the reactants of the reaction. What is a skeleton equation example. Skeletal chemical equation is a representation of a chemical reaction using chemical formulae of reactants and products.