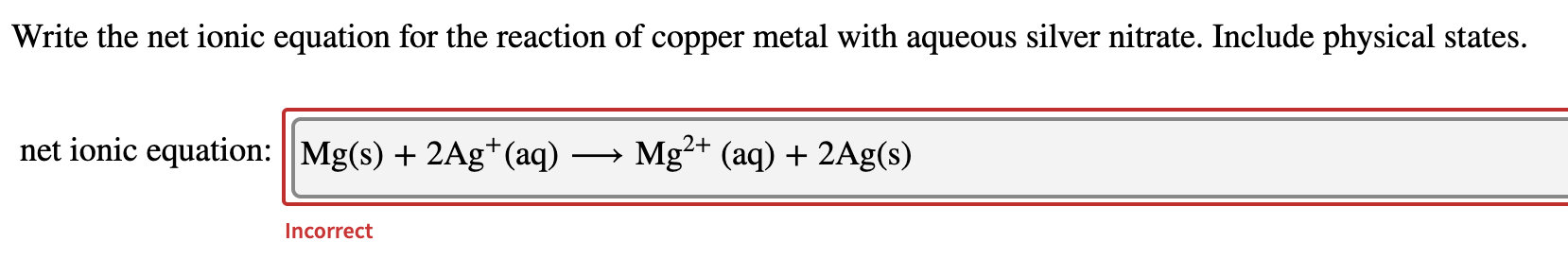Brilliant Silver Nitrate And Copper Ionic Equation

This type of reaction is called a precipitation reaction and the solid produced in the reaction is known as the precipitateYou can predict whether a precipitate will form using a list of solubility rules such as those found in the table below.
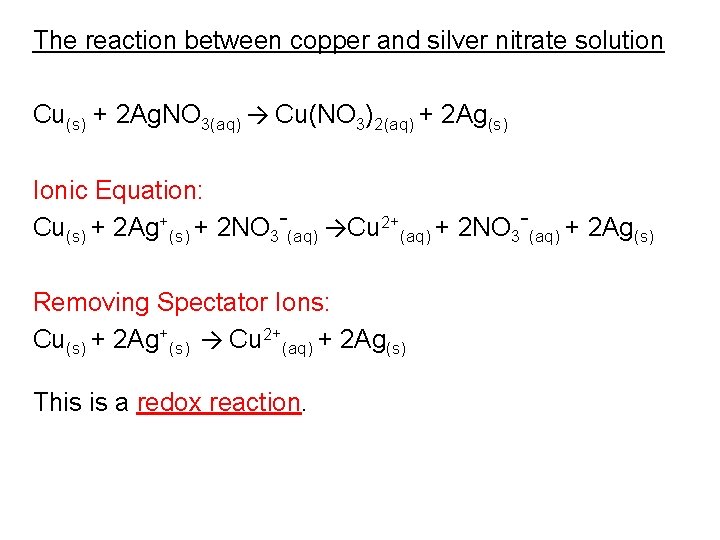
Silver nitrate and copper ionic equation. 2 AgNO3 aq Cu s --- Cu NO32 aq 2 Ag s Assumptions in this model. Honors Chemistry Name_____ Period_____ Net Ionic Equation Worksheet READ THIS. A simple demonstration of a redox reaction involves placing a solid piece of copper wire in a silver nitrate solution.
The total ionic equation would be. A common type of displacement reaction takes place when a reactive metal reacts with the salt of a less reactive metal. Cu s 2AgNO3aq 2Ag s Cu NO32aq The ionic equation includes all of the ions in the reactants and products.
The example below shows the reaction of solid magnesium metal with aqueous silver nitrate to form aqueous magnesium nitrate and silver metal. In the activity series it shows that copper is above silver so this reaction will take place. H 6 Net-ionic equation.
Calcium sulfide leadII nitrate. The balanced equation for the reaction is. Silver ions are undergoing reduction.
Total-ionic - 3 Sodium ana nitrate. Silver nitrate and copper reaction - v5 with ions This is the Base Model in Chemistry of Silver Nitrate and Copper. A neutral element would not carry a charge so it will not be a spectator ion.
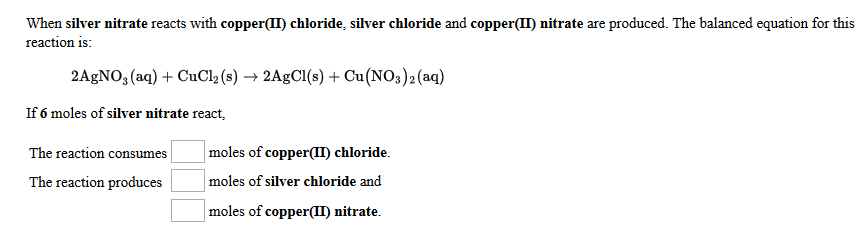
For example copper reacts with silver nitrate solution to produce silver and. Both products are soluble 3. S0270 Silver Foil 0005 2 5 g C0213 Copper II Nitrate Reagent 25 g Z0030 Zinc Nitrate Solution 10 M 500 mL M0114 Magnesium Nitrate Reagent 100 g L0067 Lead Nitrate Solution 10 M 500 mL S0304 Silver Nitrate Solution 10 M 25 mL Consult your Flinn Scientific CatalogReference Manual for current prices.