Exemplary Balanced Chemical Equation For The Combustion Of Methane

Arrange the reactions given below in proper sequence for the conversion of ethane to methane.
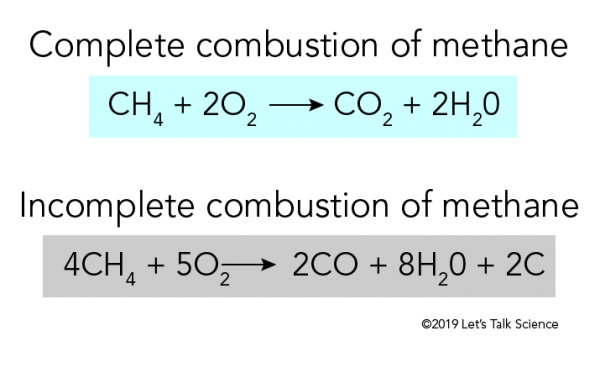
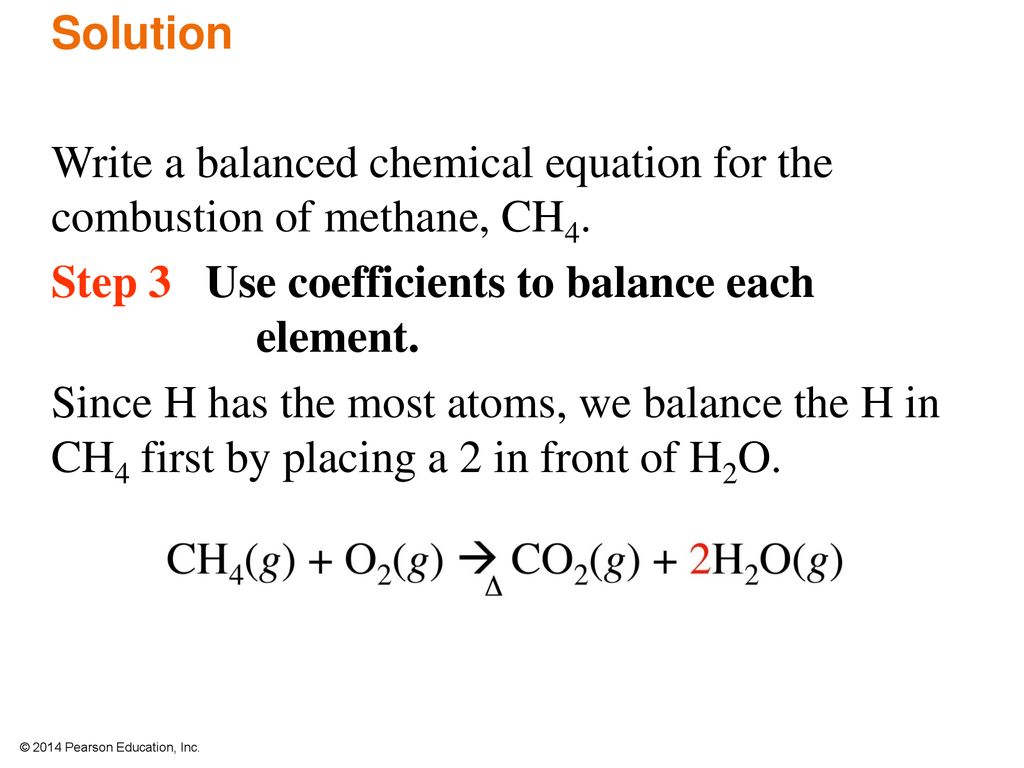
Balanced chemical equation for the combustion of methane. The combustion of methane is represented by the equation. The Combustion of Hydrocarbons - Chemistry. The combustion of methane CH4 produces carbon dioxide CO2 and water.
Regarding this what is the balanced equation for the complete combustion of methane. In the reaction the bonds in the methane and oxygen come apart the atoms rearrange and then re-bond to form water and carbon dioxide. Additionally what are the products for the combustion of methane ch4.
Express your answer as a chemical equation. When methane CH4 gas is burned in the presence of oxygen the following chemical reaction occurs. The balance equation for the complete combustion of ethane is.
Combustion is a type of reaction that involves combustible material and an oxidizer to form an oxidized reaction so it. Write a balanced equation for the combustion of methanol. Is Na cl2 a balanced equation.
All the atoms in the reactants form the products so the mass of the reactants and the products is the same. Now there are two sodium and two chlorine atoms on the right side. Calculate the volume of carbon dioxide gas that is produced.
The combustion of methane gas heats a pot on a stove. Atoms Chemical Kinetics Moving Charges and Magnetism Microbes in Human Welfare Semiconductor Electronics. CH4 2 O2 excess.