Ideal Rust Balanced Equation

The representation of a chemical reaction in the form of substances is known as a chemical equation.
Rust balanced equation. The equation in which the number of atoms of all the molecules is equal on both sides of the equation is known as a balanced chemical equation. Although its a complex process the chemical equation is simply 4Fe 3O2 6H2O 4Fe OH3. The balanced equation for the reduction of iron ore to the metal using CO is Fe 2 O 3 s 3 COg 2 Fes 3 CO 2 g a What is the maximum mass of iron in grams that can be.
Oxidation of Solid Iron Its common knowledge that rust occurs when you leave water on a metal implement or you leave it. These reactions are called combustion reactions. Metal oxygen metal oxide.
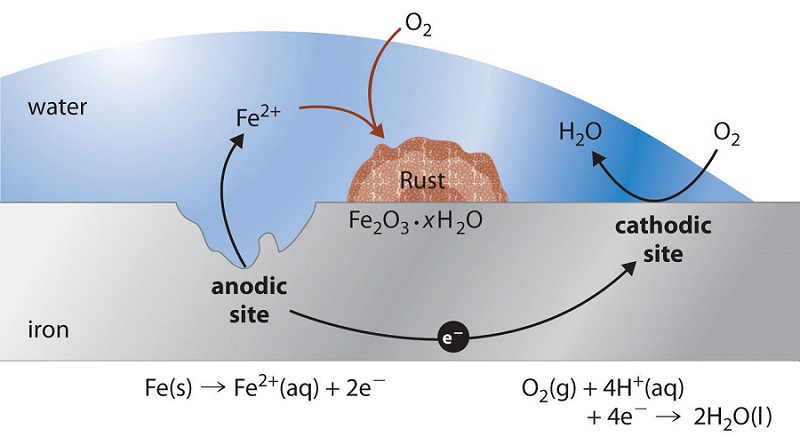
Balanced equation of rusting of iron. The general equation for this reaction is. Rusting is the corrosion of iron.
Two examples of combustion reactions are. Although its a complex process the chemical equation is simply 4Fe 3O2 6H2O 4FeOH3. Rust is the common name for iron oxideThe most familiar form of rust is the reddish coating that forms flakes on iron and steel Fe 2 O 3 but rust also comes in other colors including yellow brown orange and even greenThe different colors reflect various chemical compositions of rust.
What reacts to form rust. Rusting is a redox reaction whereby oxygen acts as the oxidising agent and iron acts as the reducing agent. One of the most common iron oxides is iron III oxide known as rust.
Oxidation of Solid Iron Its common knowledge that rust occurs when you leave water on a. Write the balanced chemical equation for this reactionExample. Rust results from a reaction called oxidation in which iron reacts with water and oxygen to form hydrated iron III oxide.




:max_bytes(150000):strip_icc():format(webp)/BalanceEquations1-56a132765f9b58b7d0bcf535.png)