Peerless Enthalpy Change Of Combustion Of Propane
And was also used for the initial development of high-accuracy ANLn composite electronic structure methods.
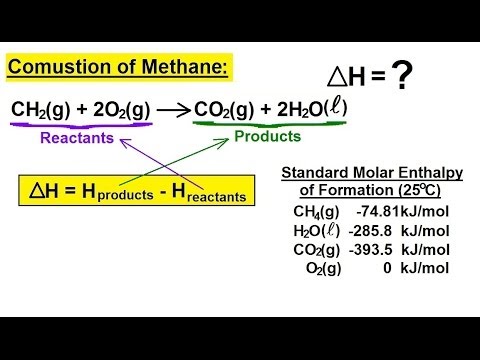
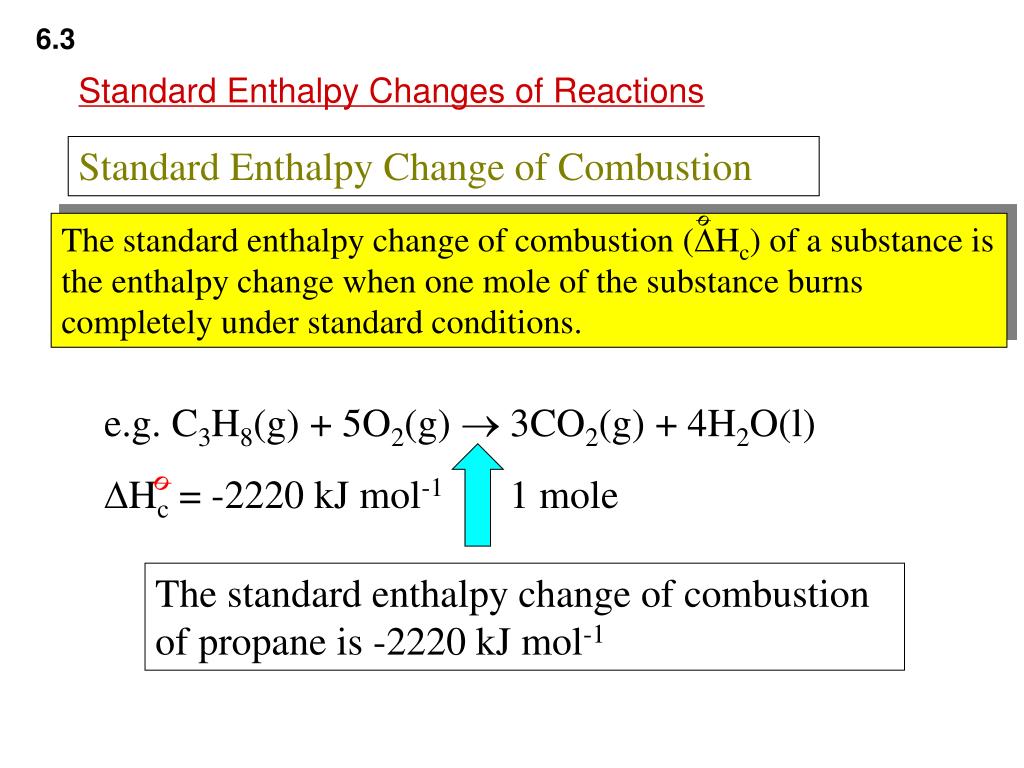
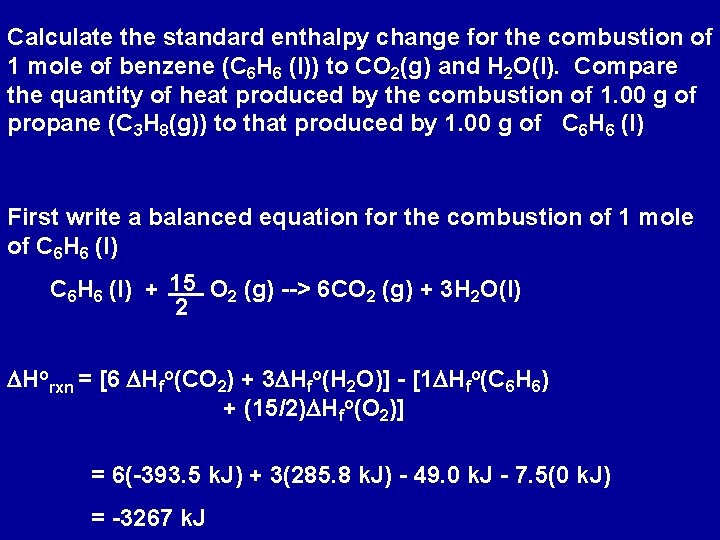
Enthalpy change of combustion of propane. ΔH Ɵ c CH 3 OH -726kJmol-1 a Write an equation including state symbols for the standard enthalpy change of combustion of methanol 2 b Write an equation including state symbols for the standard enthalpy change of formation of methanol 2 c Liquid petroleum gas is a fuel that contains propane C 3 H 8. NIST Chemistry WebBook The National Institute of Standards and Technology NIST uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific. The standard enthalpy of combustion is ΔH c.
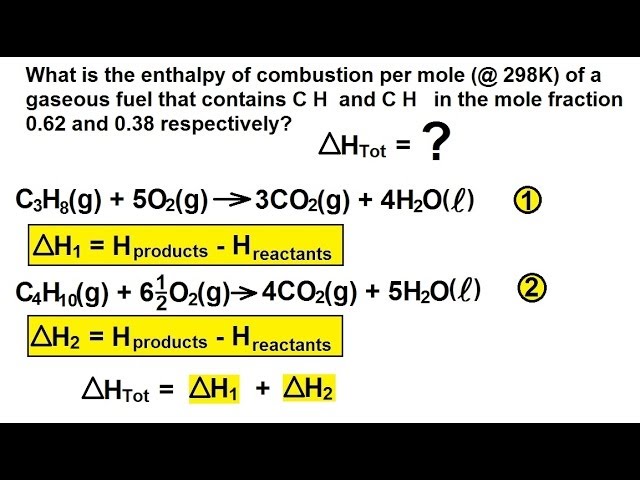
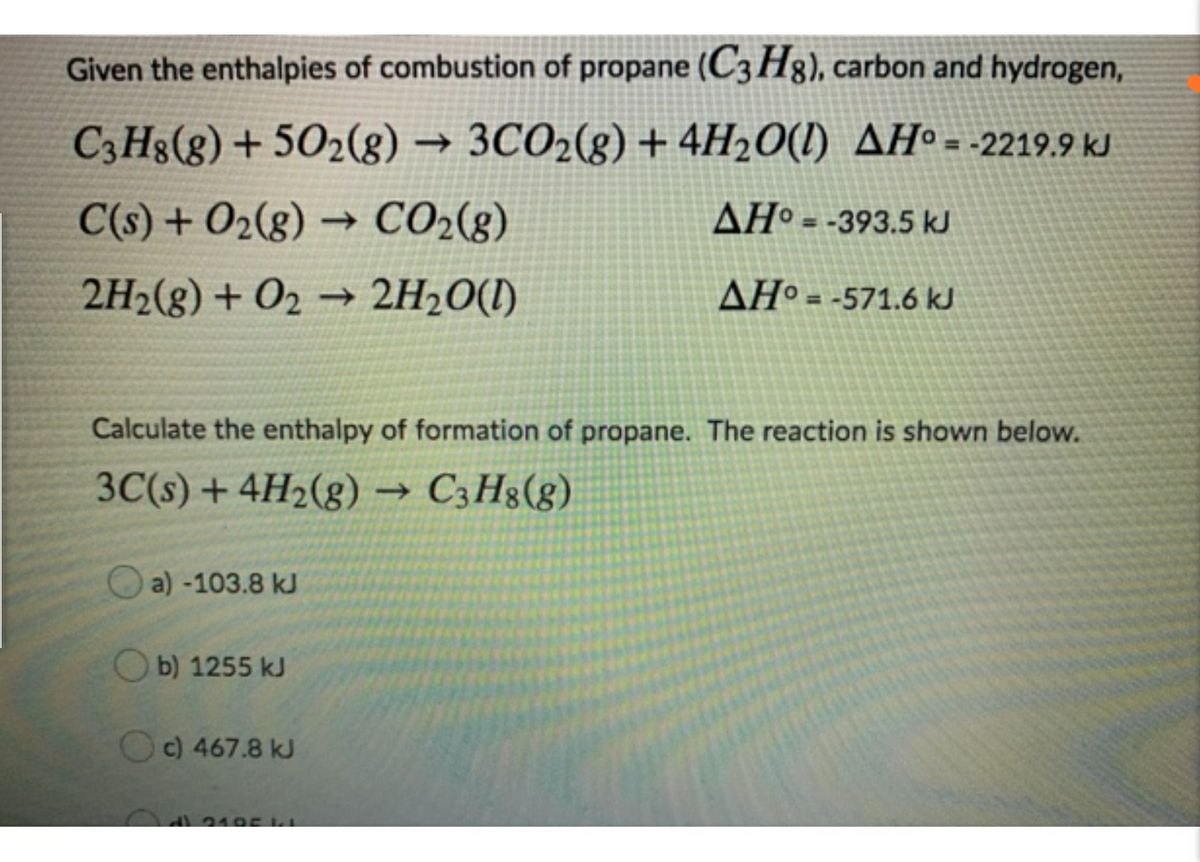
Because enthalpy is an extensive property the enthalpy change for this step is 3CO2g. The -2219 is the bond enthalpy for combustion of propane. Use the enthalpy of combustion of propene CH2 CHCH3 g Δ HCo -2058 kJ mol-1 and the fact that Δ Hfo H2O l -28583 kJ mol-1 and Δ Hfo CO2 g -39351 kJ mol-1 to find its enthalpy of formation.
It is the heat evolved when 1 mol of a substance burns completely in oxygen at standard conditions. Equation 524 is the formation reaction for 3 mol of CO2g. The enthalpy of combustion of propane C3H8 gas in terms of given data is.
Calculate a the standard enthapy and b the standard internal energy of combustion of the liquid. Similarly the enthalpy change for Equation 525 is 4H2Ol. The enthalpy of formation of propane is 104 kJmol.
The calorific value is the total energy released as heat when a substance undergoes complete combustion with oxygen under standard conditionsThe chemical reaction is typically a hydrocarbon or other. As a result the enthalpy change for this reaction is -C3H8g. You got the right answer though I still dont understand why you chuck the minus All combustion reactions are exothermic so AH is negative so you always stick in a minus at the end regardless.
Early experimental effort at providing home tuition in chemistry via YouTubeThe short method is used to draw a Hess Cycle and calculate the enthalpy of comb. 314 bond energies Author. The molar heat of combustion of propane a major constituent of liquefied petroleum gas LPG is 2220 kJ mol1.