Breathtaking Equivalent Weight Meaning In Chemistry
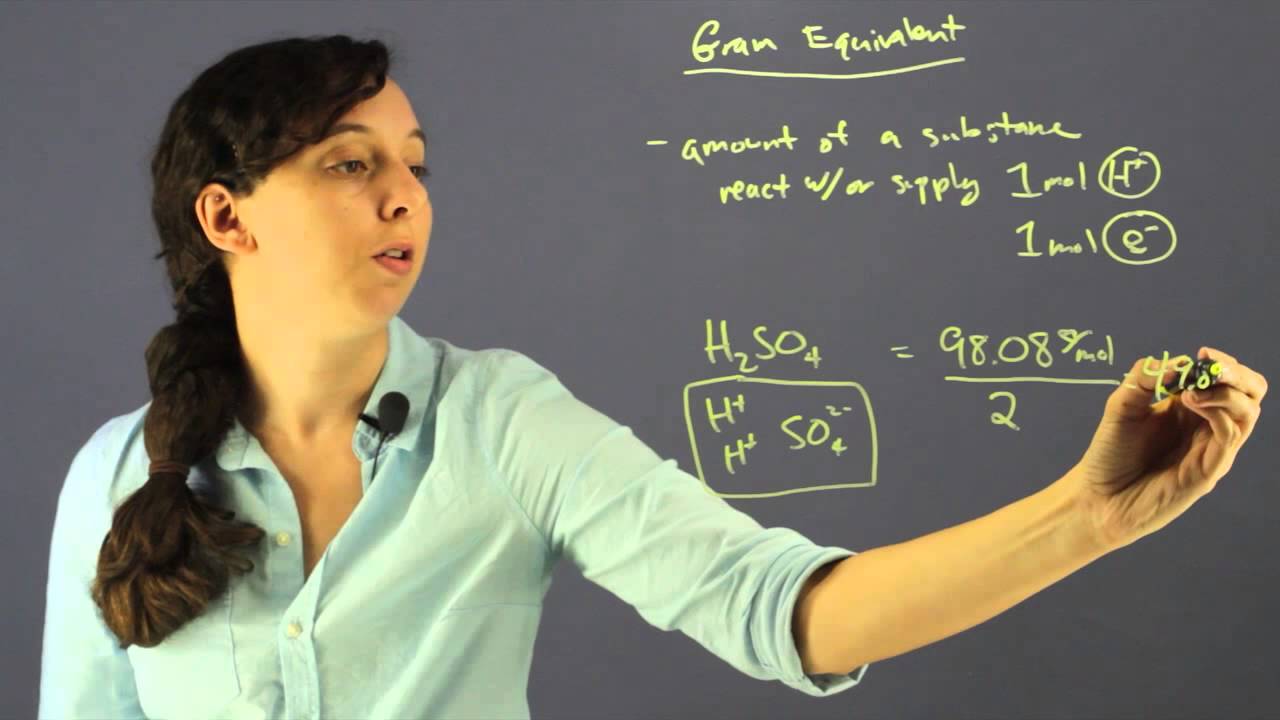
This is beacuse according to the balanced chemical reaction one mole of sulfuric acid reacts with TWO equivalents of hydroxide ie.
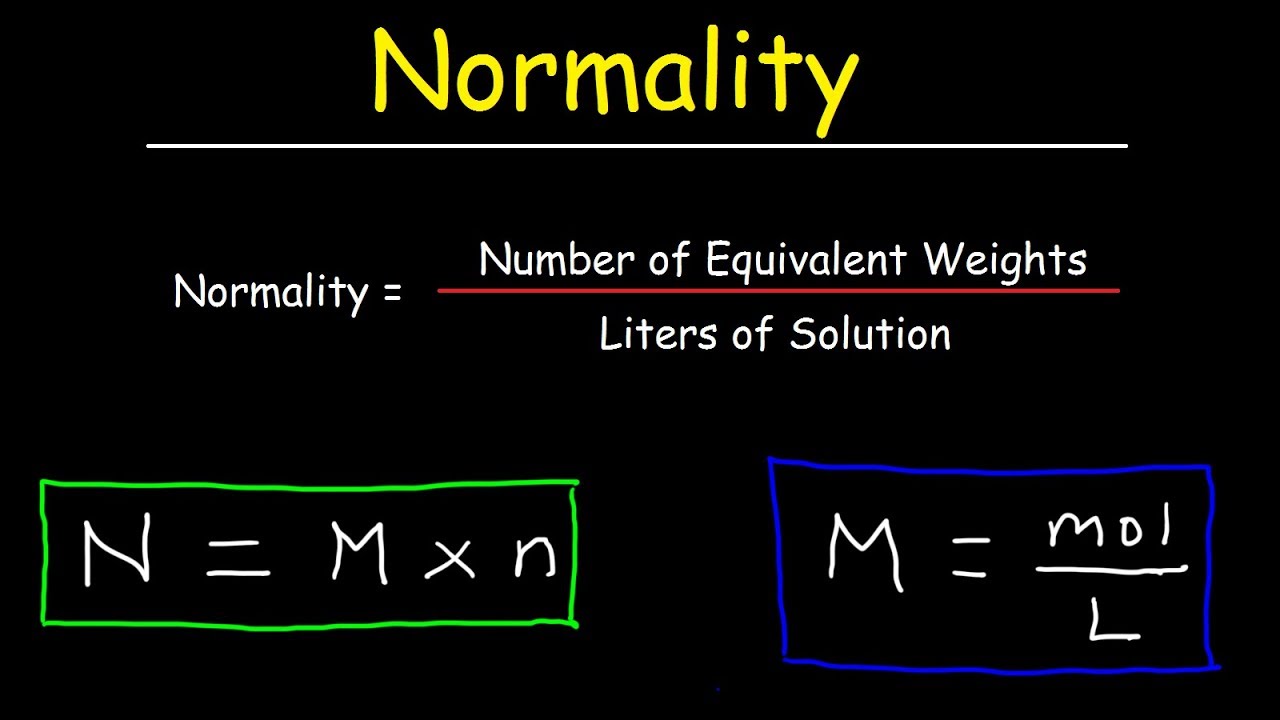
Equivalent weight meaning in chemistry. Mass is the amount of matter in something while weight is the gravitational pull on a mass. Equivalent mass equivalent weight is the mass of a substance that will combine or displace a fixed amount or units of another substance. 2Equivalent weight of an acid or a base is its formula weight divided by the number of protons it donates or accepts.
1008 parts by mass of hydrogen or 8 parts by mass of oxygen or 355 parts by mass of chlorine. The amount of substances that completely react with each other in the reaction is called equivalent weight in chemistry. The atomic or molecular weight divided by the valence.
We define the equivalent weight of a substance element or compound as. Gram equivalent and equivalent weight are important terms we use in chemical calculations. The number of parts by weight of it that will combine with or displace directly or indirectly 1008 parts by weight of hydrogen 8 parts by weight of oxygen 355 parts by weight chlorine or the equivalent parts by weight of another element.
JEE Main 2021 LIVE Chemistry Paper Solutions 24. In this example the magnitude of the equivalent weight of sulfuric acid is HALF of that of the molecular weight. When we say equivalent it is the number of moles of reactive units in a compound.
Equivalent weight definition is - the mass of a substance especially in grams that combines with or is chemically equivalent to eight grams of oxygen or one gram of hydrogen. The mass of one equivalent of a substance is called its equivalent weight. Equivalent weight is the amount of an element that reacts or is involved in reaction with 1 mole of electrons.
Equivalent weight is really not a weight it is a mass. An earlier definition used especially for chemical elements holds that an equivalent is the amount of a substance that will react with 1 g 0035 oz of hydrogen 8 g 028 oz of oxygen or 355 g 125 oz of chlorine or that will displace any of the three. Equivalent weight is the mass of number of moles of one reactant in a balanced reactionor product divided by the number of moles of another.