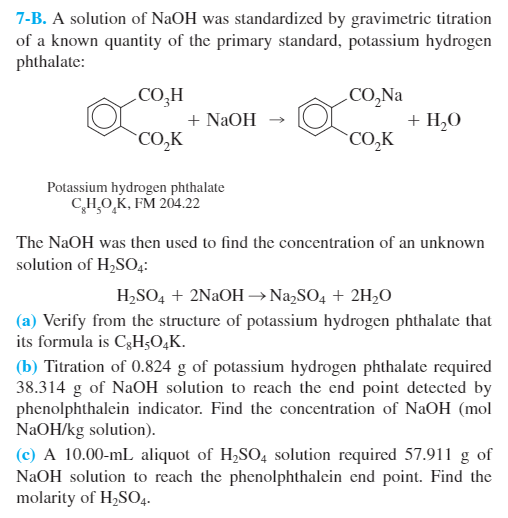
Formidable Khp And Naoh Balanced Equation
When KHP and NaOH combine a positive hydrogen ion leaves the KHC8H4O4 and a negative hydrogen atom leaves the NaOH.
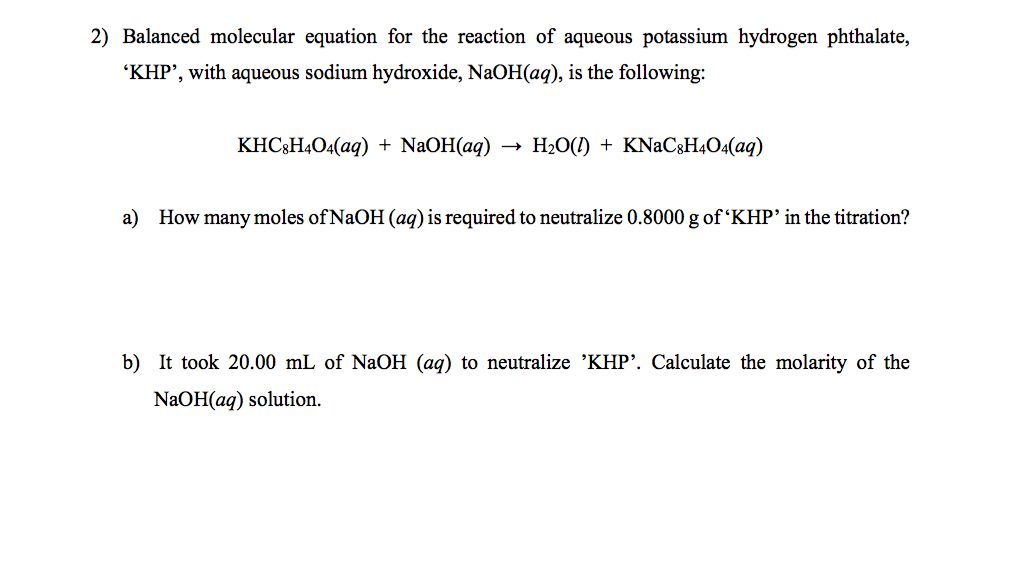
Khp and naoh balanced equation. KHC8H4O4 aq NaOH aq -- KNaC8H4O4 aq H2O l. Finally the molarity of the sodium hydroxide solution can be determined. KHP NaOH - KNaP HO Data from video.
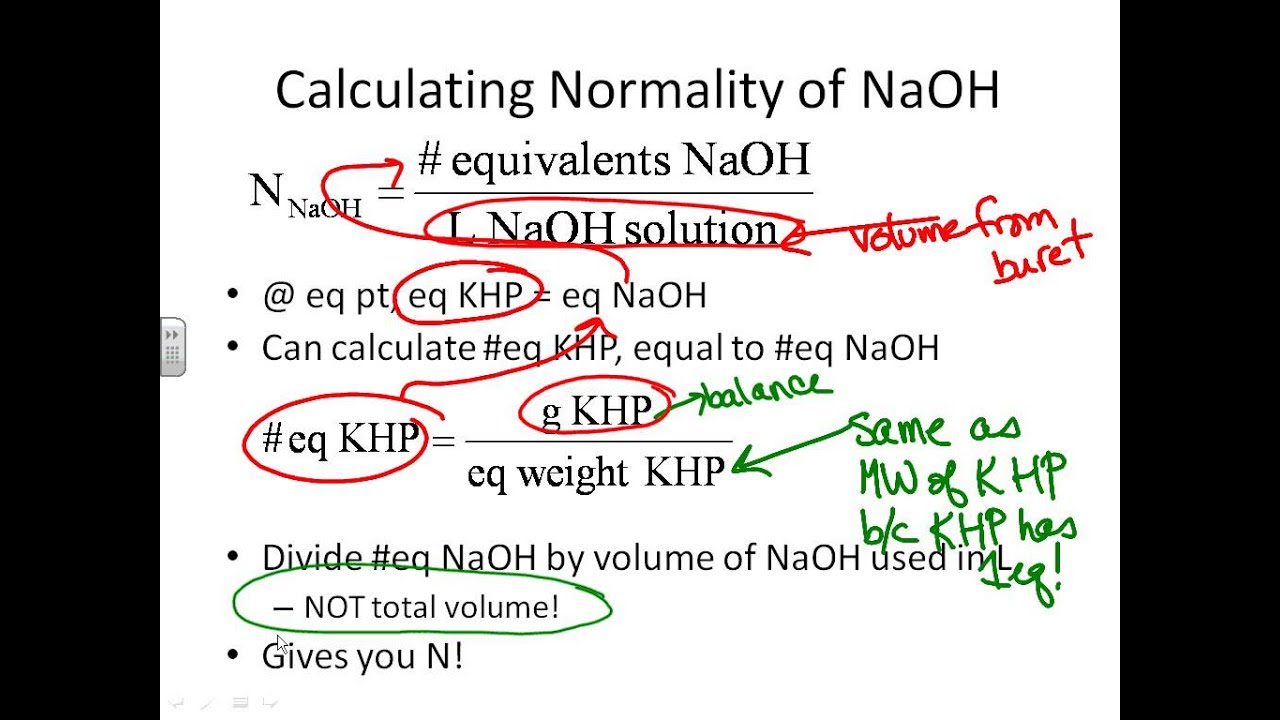
The equation is solved for MNaOH. The molar mass of KHP is approximately 20422 gmol. We have a balanced chemical equation in which we now know the exact quantity of one of the reagents KHP - NaOH aq KHC 8 H 4 O 4 aq KNaC 8 H 4 O 4 aq H 2 O l - thus we can determine the number of moles of NaOH that reacted with the KHP.
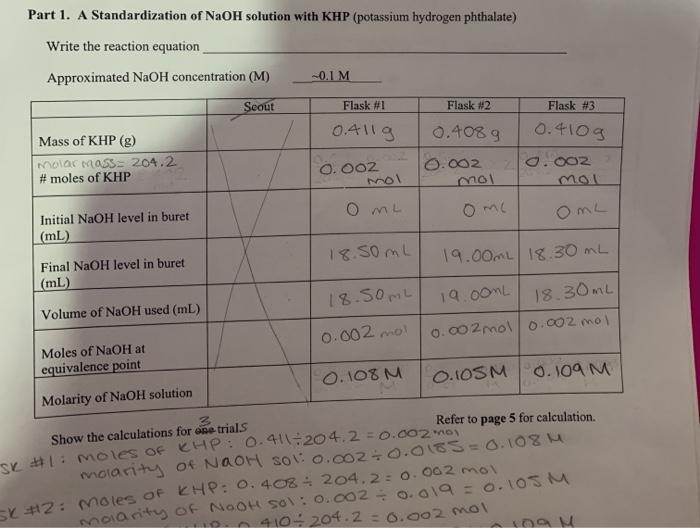
Trial 1 Trial 2 Trial 3 Volume of NaOH used 15520L 1508 1513mL Final. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Number of moles of KHP neutralized number of moles of NaOH added Restated in an equation which holds at the equivalence point.
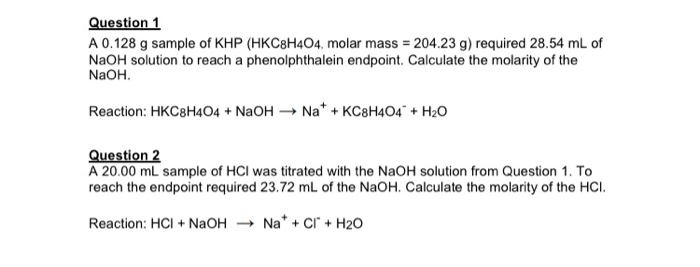
154g of KHP is equivalent to 000754 mol of KHP. HC8H4O4-1aq OH- C8H4O4-2aq aq H2Ol. These are two terms that often lead to some confusion.
KHP has one acidic hydrogen atom and reacts with NaOH on a 11 stoichiometric basis. The answer will. When NaOH is still added after the equivalence point there is excess NaOH added to KNaC 8 H.
Examples of complete chemical equations to balance. Titration Lab Balanced chemical equation for the reaction. Please tell about this free chemistry software to your friends.