Brilliant Rust Formation Equation
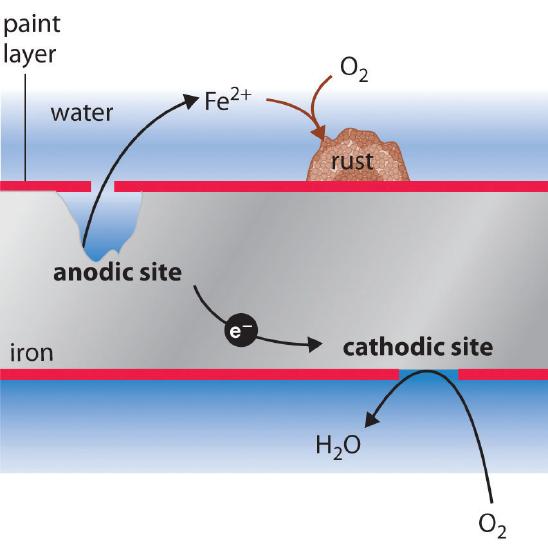
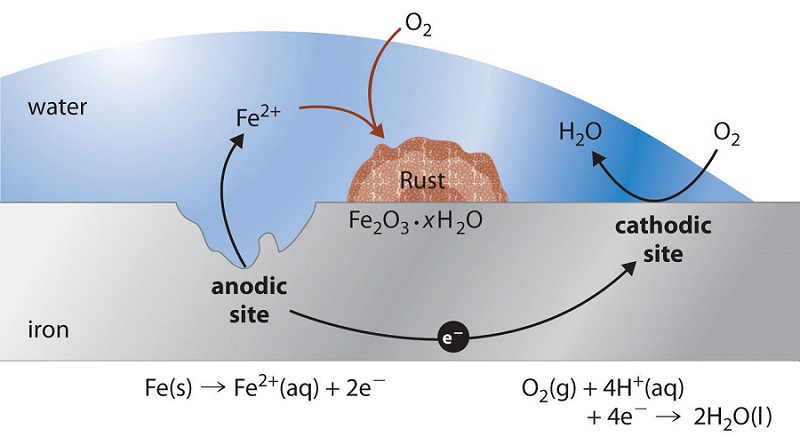
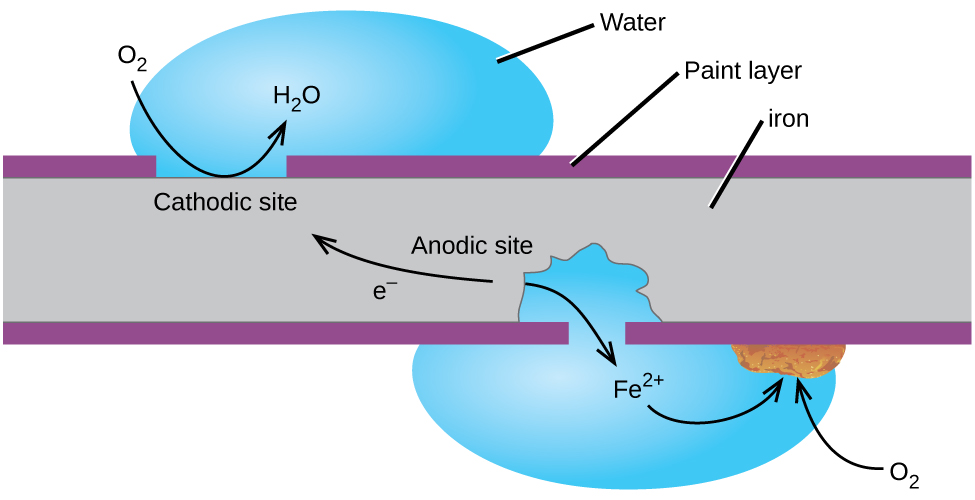
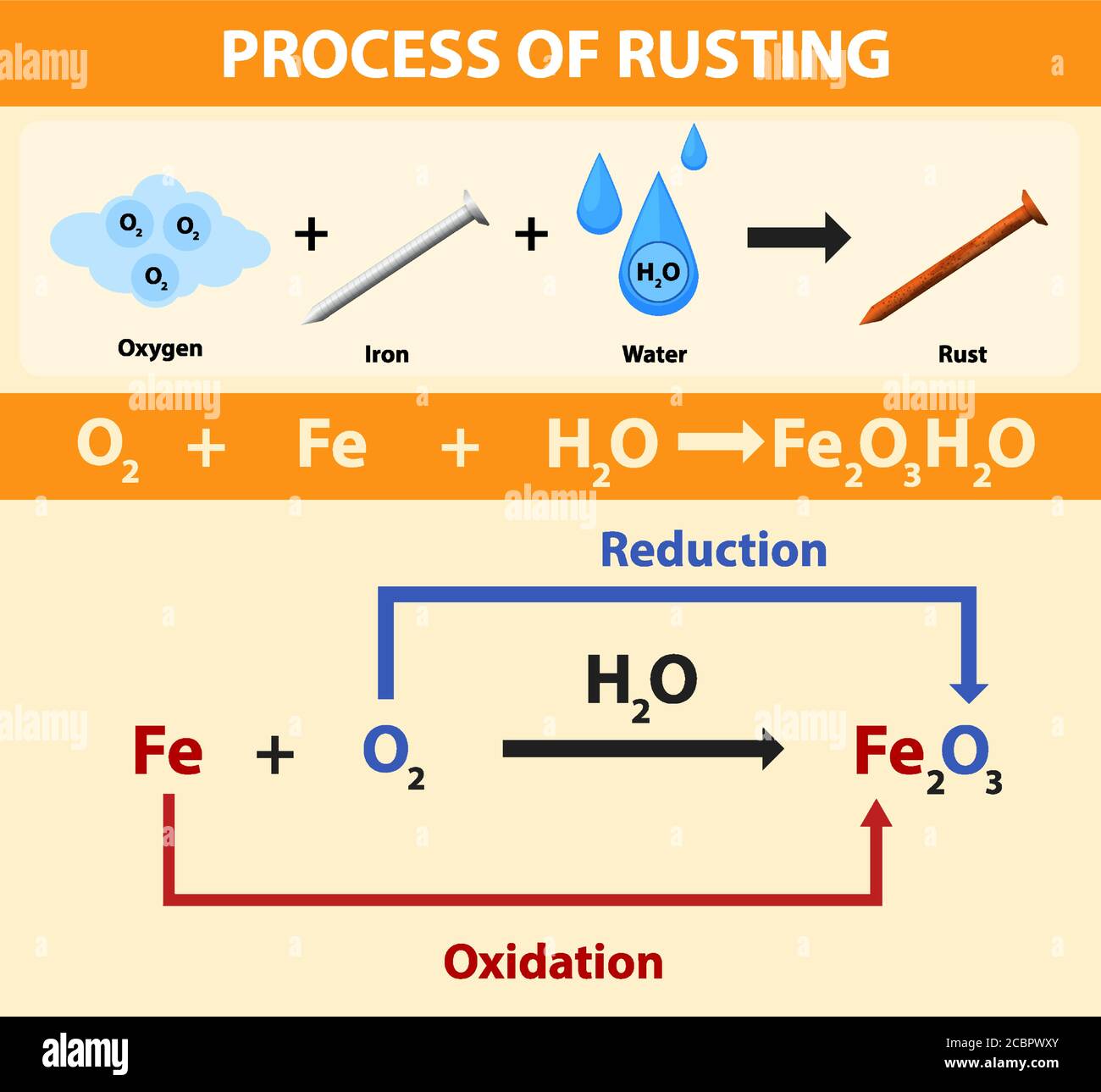
Rusting is a redox reaction whereby oxygen acts as the oxidising agent and iron acts as the reducing agent.
Rust formation equation. Periodic Properties - Common Ores of. The general equation for this reaction is. Metal oxygen metal oxide.
This rust is formed from a redox reaction between oxygen and iron in an environment containing water such as air containing high levels of moisture. Iron water oxygen hydrated iron III oxide Iron and. Two examples of combustion reactions are.
Rust is formed when iron reacts with oxygen in moist air. Think about the information that you needed on the previous page to determine that rusting is a synthesis reaction. The chemical equations for rust formation 2Fe s 2H2O l O2 g 2Fe2 aq 4OH- aq Fe2 aq 2OH- aq Fe OH2 s Fe OH2 s O2 Fe OH3 s.
It is the most common corrosion of metal around. Better learning for better results. Rust is apparently a hydrated form of iron IIIoxide.
It is an electrochemical process which requires the presence of water oxygen and an electrolyte. Here is the word equation for the reaction. Rusting is the corrosion of iron.
The rusting of iron is characterized by the formation of a layer of a red. 4Fe 3O2 g 2Fe2O3. For example solid Iron metal is has an enthalpy of formation of zero however liquid Iron although still a pure element does not since Irons natural state at 27315 Kelvin 0 C is solid.