Fun Which Equation Represents A Decomposition Reaction

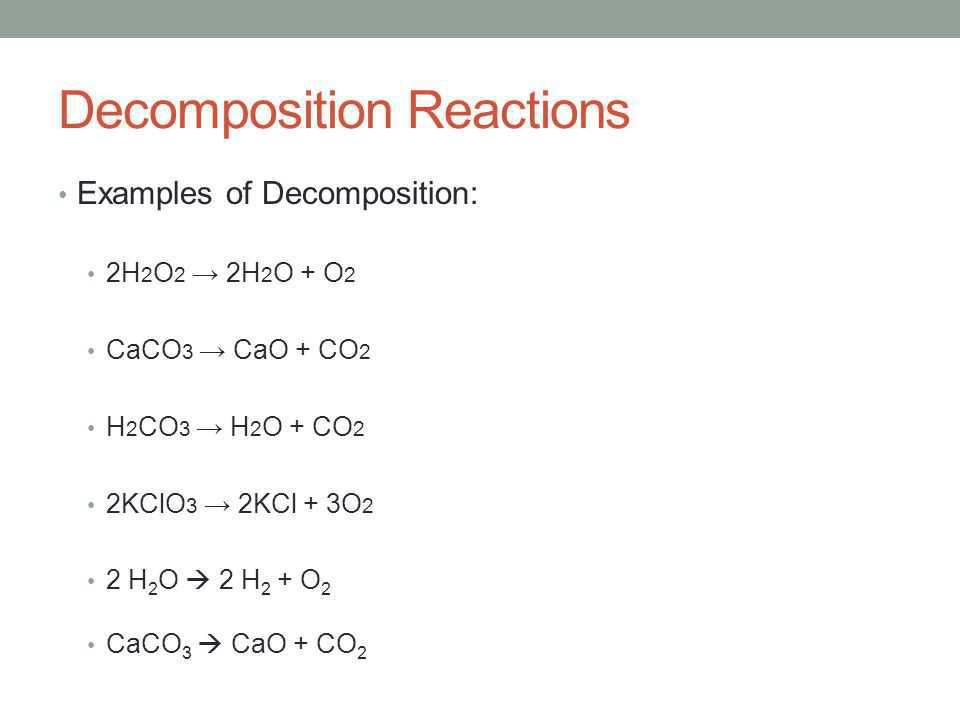
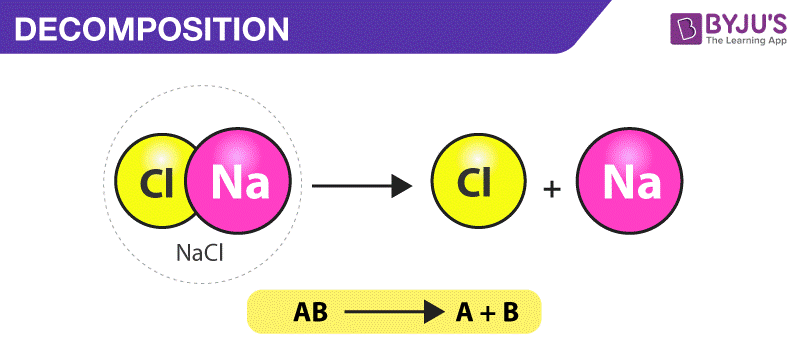
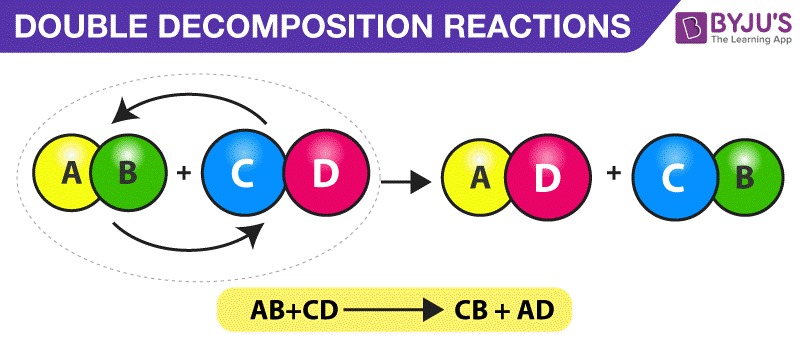
Decomposition reaction is a chemical reaction in which one reactant decomposes to form two or more products.
Which equation represents a decomposition reaction. Upon heating ferrous sulphate crystals green in color lose water as they have water of crystallization and forms anhydrous ferrous sulphate FeSO4 that is white in color. D 2 Mg O2 2 MgO. The third potential explanation is that the sodium bicarbonate decomposes into sodium oxide Na2O carbon dioxide and water when it is heated.
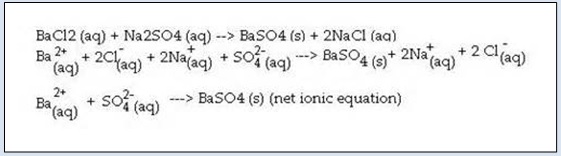
C Cd NO32 Na2S CdS 2 NaNO3. A decomposition reaction occurs when one reactant breaks down into two or more products. Thus as the initial concentration increases the half-life increases.
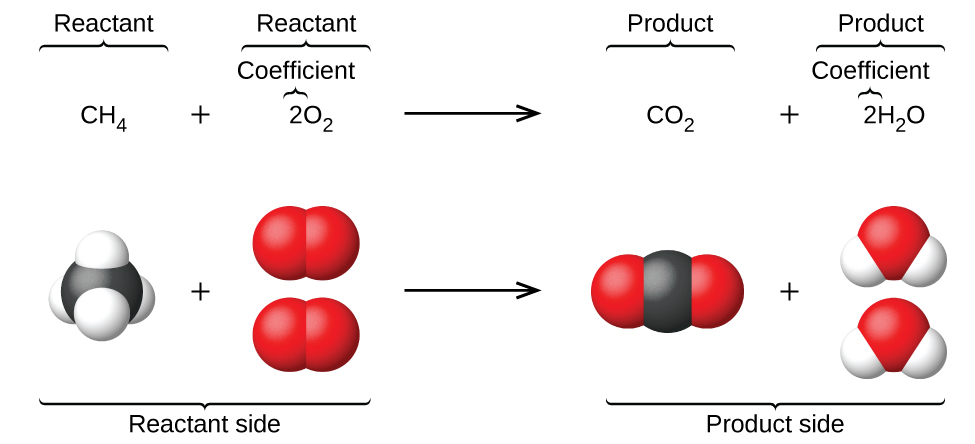
Which chemical equation correctly represents the decomposition reaction that takes place when ammonia breaks down to form hydrogen gas and nitrogen gas. 1 question Which equation represents a decomposition reaction. 2H2O 2H2 O2.
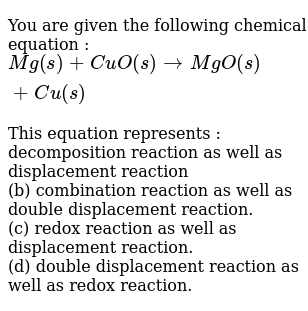
Given the lead-acid battery reaction. B energy in the form of heat or light is often produced. Chemistry 2 - Types of Reactions.
Which equation represents a decomposition reaction. View the answer now. Which equation represents a decomposition reaction.
The balanced chemical equation for this potential reaction is 2NaHCO3s Na2Os 2CO2g H2Og. An electrolytic decomposition reaction is a type of decomposition reaction in which the activation energy for decomposition is provided in the form of electrical energy. An example of an electrolytic decomposition reaction is the electrolysis of water which can be represented by the following chemical equation.