Ideal Butane And Oxygen Reaction

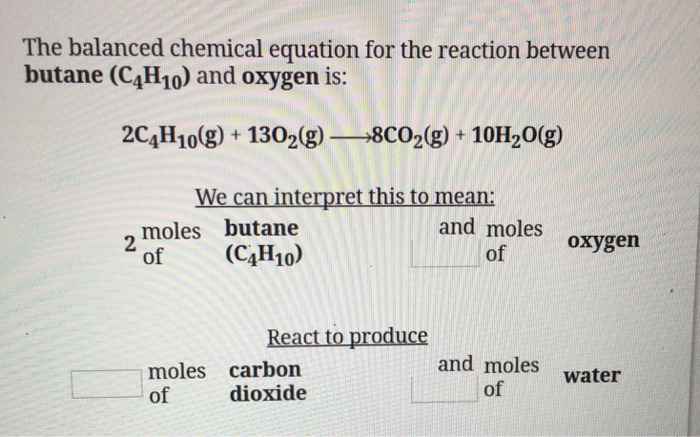
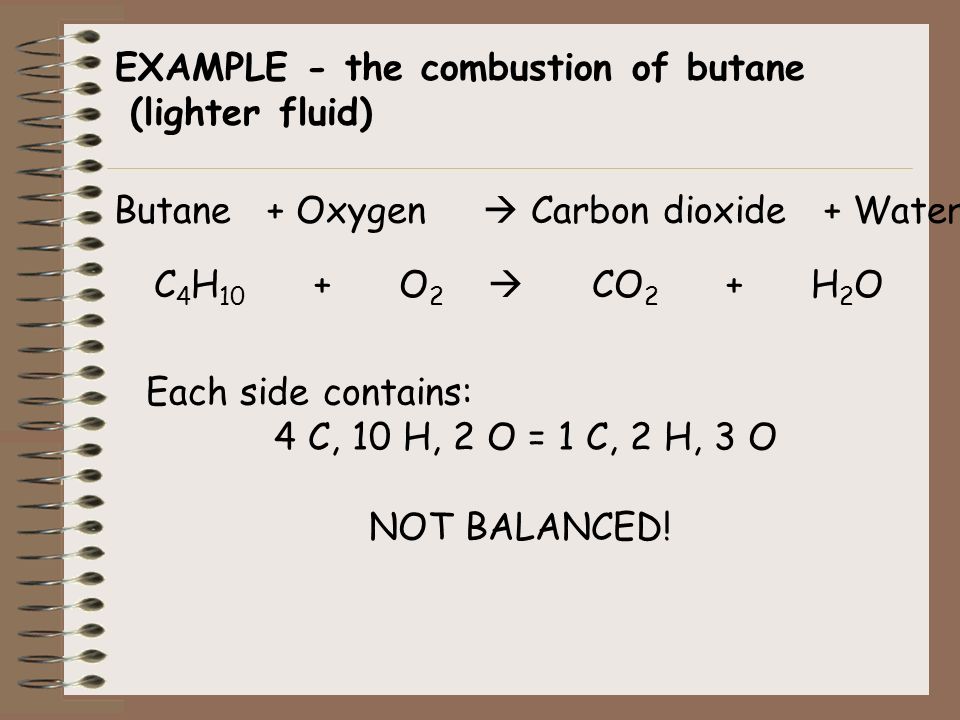
Gaseous butane CH3CH22CH3 will react with gaseous oxygen O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O.
Butane and oxygen reaction. The anodic reaction requires a catalyst that contains expensive precious metals. It will consume first in the balanced equation. The balanced equation for the combustion of butane combines two molecules of butane with 13 oxygen molecules.
M 800 gm of oxygen will react here 232 gm of Butane will react with 800 gm of oxygen to form the CO2 and H2O products. So Butane is the limiting reagent. CH3OH 32 O2CO2 2 H2O The electrochemical reaction produces carbon dioxide a greenhouse gas.
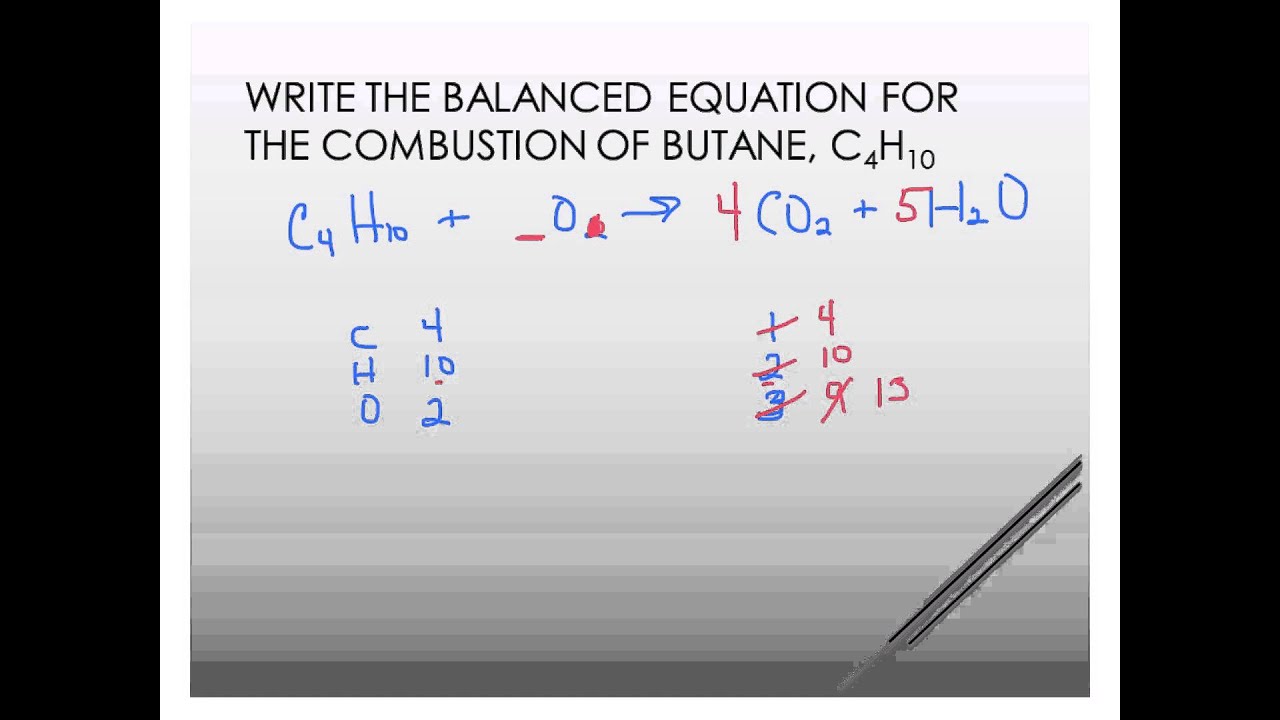
The combustion of butane is a reaction between butane and oxygen gas that produces carbon dioxide gas and water. In fact for every two moles of butane. A mixture of ruthenium and palladium is typical.
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor gas. M 232 gm of Butane will react Molar mass of Oxygen 32 gmol. Depending upon the attachment of carbon atoms butane exhibits structural isomerism eg isobutane and n-butane.
The combination produces eight molecules of carbon dioxide and 10 water molecules. M 32 x 25. There is a barrier to the reaction.
What is the balanced equation when ethane reacts with oxygen. 2C4H10 13O2 8CO2 10H2O Use stoichiometry to calculate mol O2 required to react with 5 mol C4H10. If you mix butane and oxygen in the correct stoichiometric ratio and if the total pressure of the mixture is 436 mmHg what is the pressure in mmHg of water vapor after the reaction.