Peerless Describe The Changes That Happen In Iron When They Are Exposed In Oxygen

The oxidation of the iron in a ferromagnesian silicate starts with the dissolution of the iron.
Describe the changes that happen in iron when they are exposed in oxygen. One erythrocyte contains four iron ions and because of this each erythrocyte is capable of carrying up to four molecules of oxygen. The reaction of iron with oxygen. We know that the iron changes into iron oxide when it is exposed.
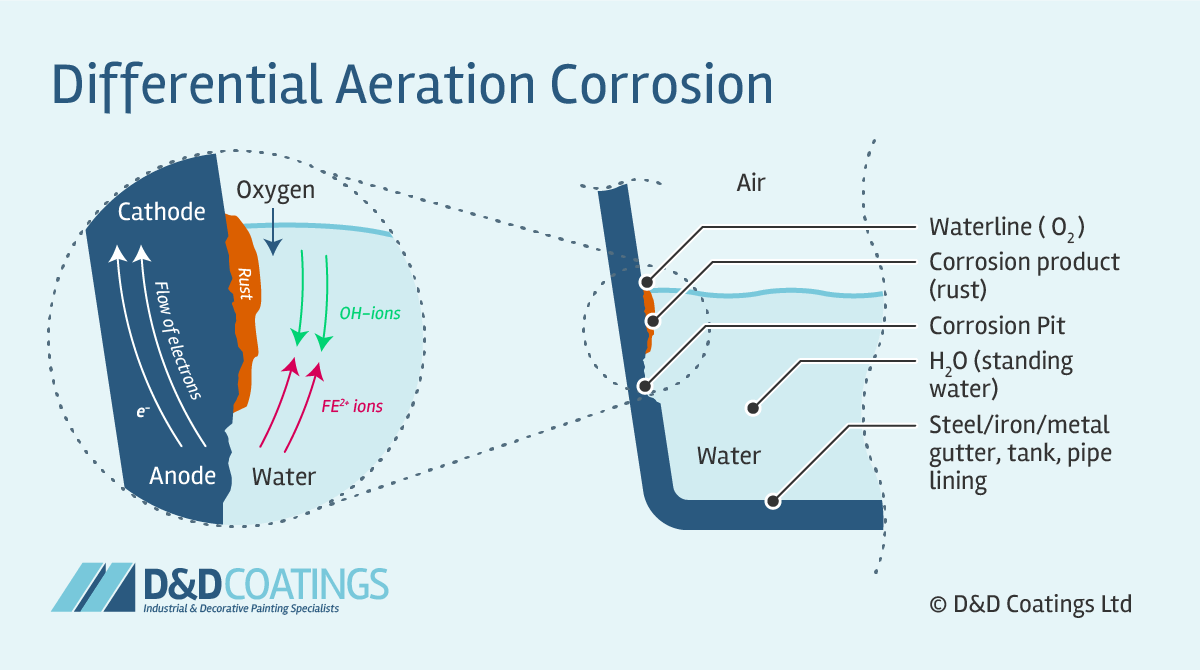
If the metal is iron we call this change rusting and the weaker flaky brown compound that is formed is rust. Effects as a function of altitude. This chemical process can occur either in the air or after the metal is exposed to water or acids.
Ferrous iron and oxygen but they do not necessarily apply to bicarbonate-con-taining waters. Check the related links if you are concerned for more. Describe the changes that happen in iron nails when they are exposed in oxygen.
This form is called oxyhemoglobin. It is very harmful as it destructs the metal completely by slowly eating up the metal. Describe the change when silver is exposed to oxygen.
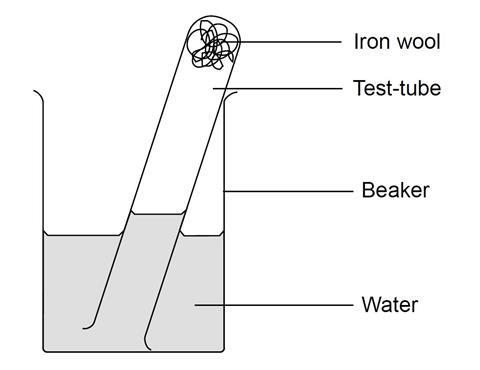
First of all the heme changes shape. In an oxidation reaction electrons are transferred from one substance to another. What do you call the process of exposing the iron nails placed in wet cloth after 48 hours that resulted to change in appearance of iron nails.
For olivine the process looks like this where olivine in the presence of carbonic acid is converted to dissolved iron carbonate and silicic acid. The most common example is the corrosion of steel which is a transformation of the iron molecules on steels surface into iron oxides most often Fe 2 O 3 and Fe 3 O 4. The electrons of iron are transferred to oxygen when iron is found in the presence of oxygen.