First Class How Will You Justify That Rusting Of Iron Is A Chemical Change

32k 645k 159.
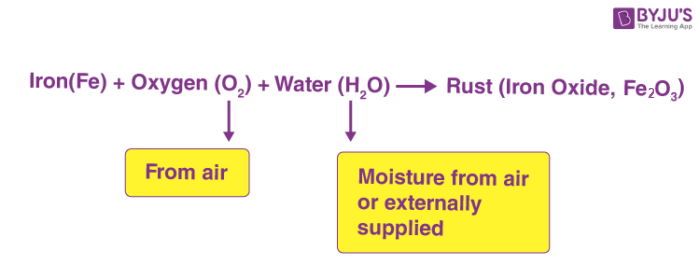
How will you justify that rusting of iron is a chemical change. The presence of oxygen and water or water vapor is essential for rusting. In these circumstances we break iron-iron bonds and oxygen-oxygen bonds and form F eIIO and F eIIIO bonds. A chemical property of iron is that it is capable of combining with oxygen to.
False Rusting of iron is a chemical change in which iron gets corroded in humid air. Hence rusting of iron is a chemical change. Rust is clearly a substance that is different from iron.
Corrosion and it is a physical as well as chemical change B. The rusting of ion is a chemical change because the iron atoms form chemical bonds with the oxygen atoms to form iron oxide molecules. Rust is another name for iron oxide which occurs when iron or an alloy that contains iron like steel is exposed to oxygen and moisture for a long period of time.
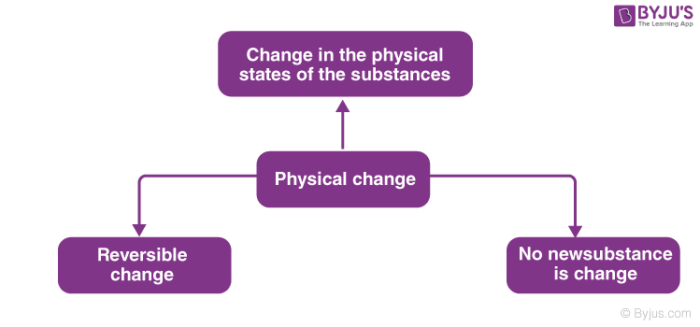
A change in which one or more new substances are formed is known as chemical change. Galvanizing Galvanizing a metal object means to coat the surface of that object with a layer of metallic zinc. A new substance formed out of the reaction.
For Example when the iron is exposed to air and moisture rust formation takes place. Dissolution and it is a chemical change. Dissolution and it is a physical change C.
A chemical change results from a chemical reaction while a physical change is when matter changes forms but not chemical identity. If the pH of the environment surrounding the metal is low the rusting process is quickened. The rusting of iron speeds up when it is exposed to acid rains.